Vsepr Theory Homework
ADVERTISEMENT
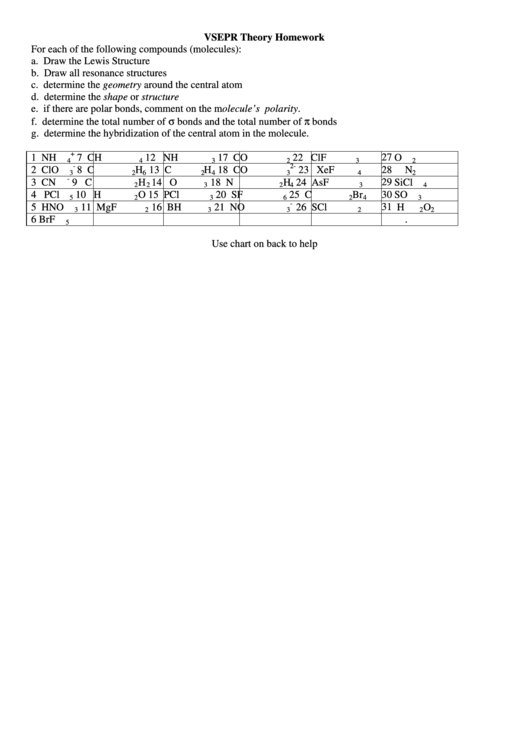
VSEPR Theory Homework
For each of the following compounds (molecules):
a. Draw the Lewis Structure
b. Draw all resonance structures
c. determine the geometry around the central atom
d. determine the shape or structure
e. if there are polar bonds, comment on the molecule’s polarity.
f. determine the total number of σ bonds and the total number of π bonds
g. determine the hybridization of the central atom in the molecule.
+
1
NH
7
CH
12
NH
17
CO
22
ClF
27
O
4
4
3
2
3
2
-
2-
2
ClO
8
C
H
13
C
H
18
CO
23
XeF
28
N
3
2
6
2
4
3
4
2
-
3
CN
9
C
H
14
O
18
N
H
24
AsF
29
SiCl
2
2
3
2
4
3
4
4
PCl
10
H
O
15
PCl
20
SF
25
C
Br
30
SO
5
2
3
6
2
4
3
-
5
HNO
11
MgF
16
BH
21
NO
26
SCl
31
H
O
3
2
3
3
2
2
2
6
BrF
.
5
Use chart on back to help
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








