Geometric Isomerism, Chemguide - Questions
ADVERTISEMENT
C h e m g u i d e – q u e s t i o n s
GEOMETRIC ISOMERISM
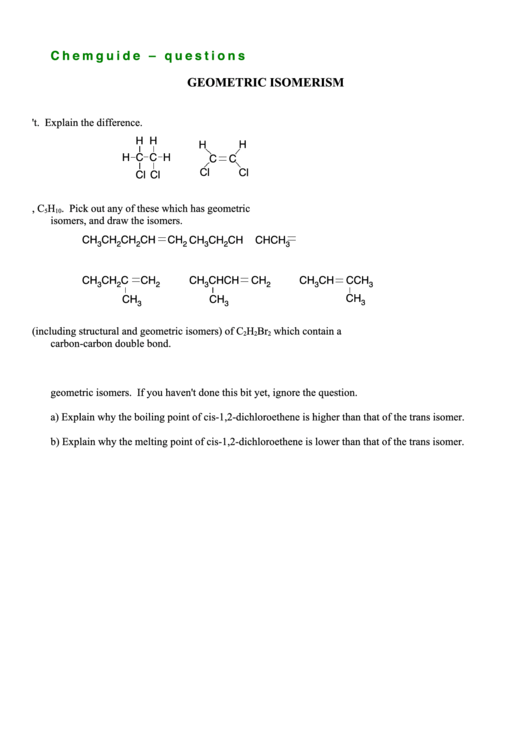
1. One of these molecules has geometric isomers and the other one doesn't. Explain the difference.
H H
H
H
H C C H
C
C
Cl
Cl
Cl
Cl
2. The following are all isomers of pentene, C
H
. Pick out any of these which has geometric
5
10
isomers, and draw the isomers.
CH
CH
CH
CH
CH
CH
CH
CH
CHCH
3
2
2
2
3
2
3
CH
CH
C
CH
CH
CHCH
CH
CH
CH
CCH
3
2
2
3
2
3
3
CH
CH
CH
3
3
3
3. Draw all the isomers (including structural and geometric isomers) of C
H
Br
which contain a
2
2
2
carbon-carbon double bond.
4. This question is about the effect of geometric isomerism on the melting and boiling points of
geometric isomers. If you haven't done this bit yet, ignore the question.
a) Explain why the boiling point of cis-1,2-dichloroethene is higher than that of the trans isomer.
b) Explain why the melting point of cis-1,2-dichloroethene is lower than that of the trans isomer.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1