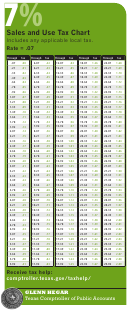
Chem 101 - Useful Information Chart
ADVERTISEMENT
Chem 101 Useful Information
1 Kg = 1000 g
1 cal = 4.184 J
1 L = 1000 mL
T(°C) + 273 = T(K)
T(°F) = 1.80(T°C) + 32
0° C = 273 K
Specific heat of water = 1 cal/g/°C
1 mol of gas at STP = 22.4 Liters
Standard Temperature and Pressure = 0° C
1 atmosphere = 760 torr
and 1 atmosphere
(1 atm = 760 mm Hg)
1 atm = 14.70 psi
R = 0.08206 L atm/mol K
Ideal Gas Law: PV = nRT
P
V
= P
V
1
1
2
2
P
V
P
V
P
P
1
1
2
2
1
2
=
=
T
T
T
T
1
2
1
2
C = λν
V
V
1
2
=
8
T
T
C = 3.00 x 10
m/s
1
2
E = hν
23
Avogadro's number = 6.022 x 10
-34
h = 6.626 x 10
J•s
Activity Series for Metals
Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Co, Ni, Sn, Pb, H, Cu, Ag, Hg, Pt, Au
Most Active
Least Active
XI
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
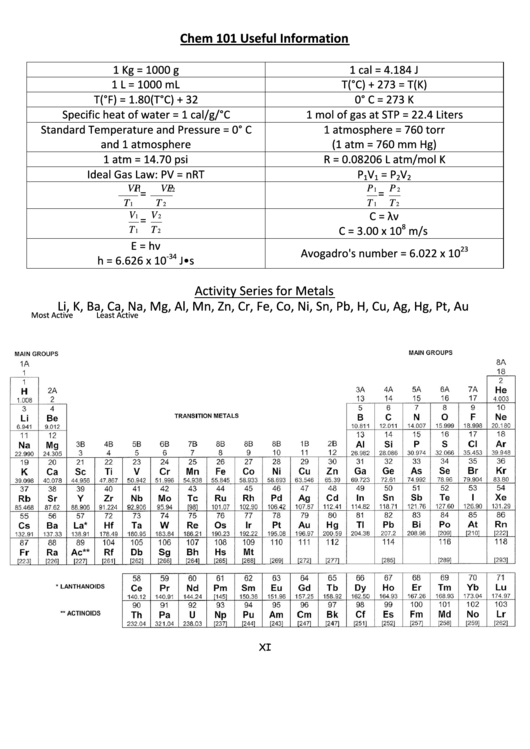 1
1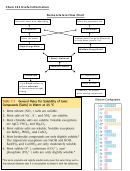 2
2