Vsepr Theory Summary Chart
ADVERTISEMENT
BLM 2.1.8B
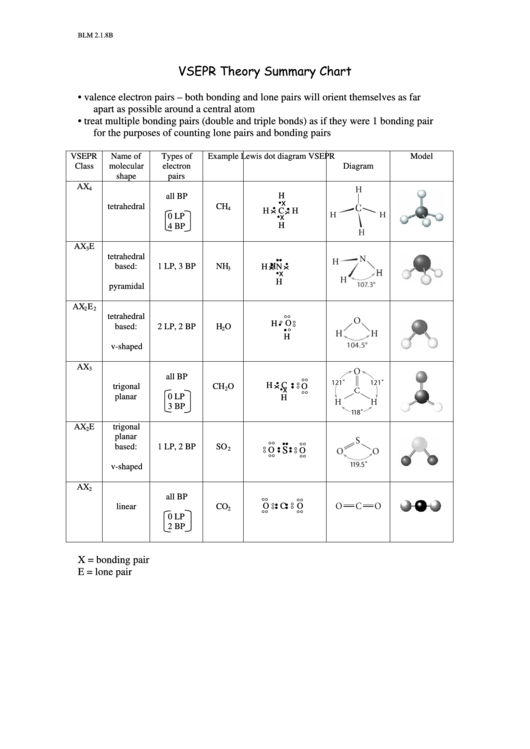
VSEPR Theory Summary Chart
•
valence electron pairs – both bonding and lone pairs will orient themselves as far
apart as possible around a central atom
•
treat multiple bonding pairs (double and triple bonds) as if they were 1 bonding pair
for the purposes of counting lone pairs and bonding pairs
VSEPR
Name of
Types of
Example
Lewis dot diagram
VSEPR
Model
Class
molecular
electron
Diagram
shape
pairs
AX
4
H
all BP
•x
tetrahedral
CH
4
H
C
H
0 LP
•x
H
4 BP
AX
E
3
tetrahedral
••
H
based:
1 LP, 3 BP
NH
H
N
3
•x
H
pyramidal
AX
E
2
2
tetrahedral
°°
O
H
based:
2 LP, 2 BP
H
O
•
2
° •
H
v-shaped
AX
3
all BP
°°
H C
trigonal
CH
O
O
2
•x
°°
planar
0 LP
H
3 BP
AX
E
trigonal
2
planar
••
°°
°°
based:
1 LP, 2 BP
SO
S
2
O
O
°°
°°
v-shaped
AX
2
all BP
°°
°°
O
C
O
linear
CO
2
°°
°°
0 LP
2 BP
X = bonding pair
E = lone pair
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








