Ap Chemistry Ion List
ADVERTISEMENT
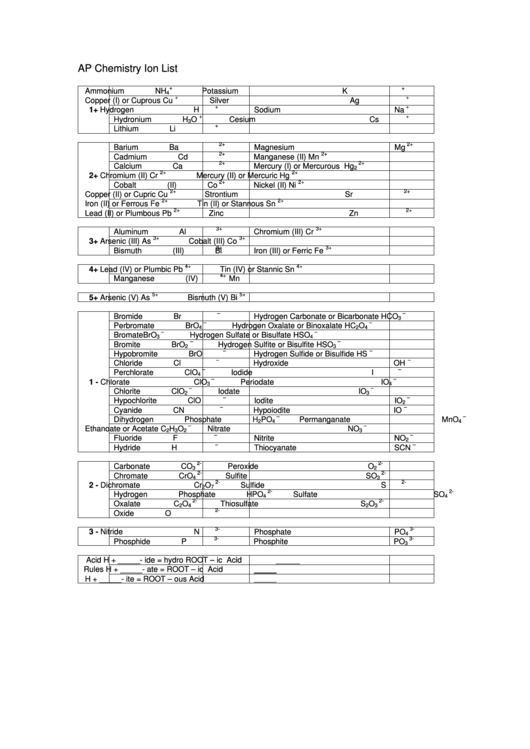
AP Chemistry Ion List
+
+
Ammonium
NH
Potassium
K
4
+
+
Copper (I) or Cuprous
Cu
Silver
Ag
+
+
1+
Hydrogen
H
Sodium
Na
+
+
Hydronium
H
O
Cesium
Cs
3
+
Lithium
Li
2+
2+
Barium
Ba
Magnesium
Mg
2+
2+
Cadmium
Cd
Manganese (II)
Mn
2+
2+
Calcium
Ca
Mercury (I) or Mercurous
Hg
2
2+
2+
2+
Chromium (II)
Cr
Mercury (II) or Mercuric
Hg
2+
2+
Cobalt (II)
Co
Nickel (II)
Ni
2+
2+
Copper (II) or Cupric
Cu
Strontium
Sr
2+
2+
Iron (II) or Ferrous
Fe
Tin (II) or Stannous
Sn
2+
2+
Lead (II) or Plumbous
Pb
Zinc
Zn
3+
3+
Aluminum
Al
Chromium (III)
Cr
3+
3+
3+
Arsenic (III)
As
Cobalt (III)
Co
3+
3+
Bismuth (III)
Bi
Iron (III) or Ferric
Fe
4+
4+
4+
Lead (IV) or Plumbic
Pb
Tin (IV) or Stannic
Sn
4+
Manganese (IV)
Mn
5+
5+
5+
Arsenic (V)
As
Bismuth (V)
Bi
–
–
Bromide
Br
Hydrogen Carbonate or Bicarbonate
HCO
3
–
–
Perbromate
BrO
Hydrogen Oxalate or Binoxalate
HC
O
4
2
4
–
–
Bromate
BrO
Hydrogen Sulfate or Bisulfate
HSO
3
4
–
–
Bromite
BrO
Hydrogen Sulfite or Bisulfite
HSO
2
3
–
–
Hypobromite
BrO
Hydrogen Sulfide or Bisulfide
HS
–
–
Chloride
Cl
Hydroxide
OH
–
–
Perchlorate
ClO
Iodide
I
4
–
–
1 -
Chlorate
ClO
Periodate
IO
3
4
–
–
Chlorite
ClO
Iodate
IO
2
3
–
–
Hypochlorite
ClO
Iodite
IO
2
–
–
Cyanide
CN
Hypoiodite
IO
–
–
Dihydrogen Phosphate
H
PO
Permanganate
MnO
2
4
4
–
–
Ethanoate or Acetate
C
H
O
Nitrate
NO
2
3
2
3
–
–
Fluoride
F
Nitrite
NO
2
–
–
Hydride
H
Thiocyanate
SCN
2-
2-
Carbonate
CO
Peroxide
O
3
2
2-
2-
Chromate
CrO
Sulfite
SO
4
3
2-
2-
2 -
Dichromate
Cr
O
Sulfide
S
2
7
2-
2-
Hydrogen Phosphate
HPO
Sulfate
SO
4
4
2-
2-
Oxalate
C
O
Thiosulfate
S
O
2
4
2
3
2-
Oxide
O
3-
3-
3 -
Nitride
N
Phosphate
PO
4
3-
3-
Phosphide
P
Phosphite
PO
3
Acid
H + _____- ide
=
hydro ROOT – ic
Acid
Rules
H + _____- ate
=
ROOT – ic
Acid
H + _____- ite
=
ROOT – ous
Acid
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








