Solubility Curve Problems Chemistry Paper
ADVERTISEMENT
Solubility Curve Problems
1. What explain why solids become
more soluble as temperature
increases and why gasses become
less soluble? (You don't need the
graph for this one.)
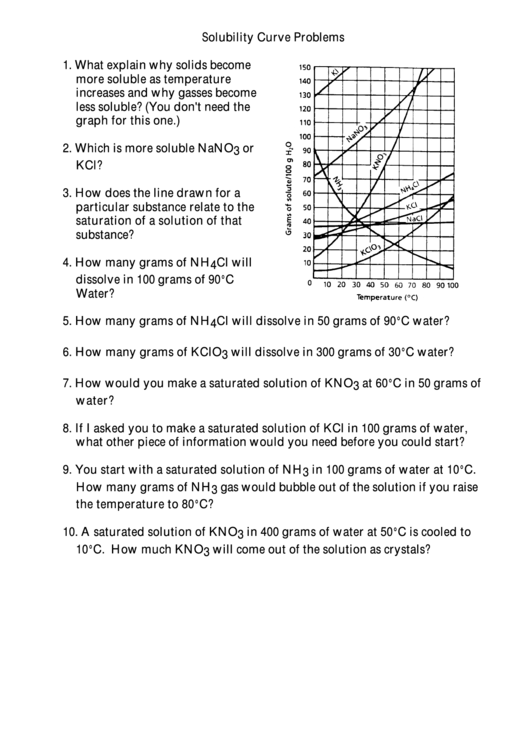
2. Which is more soluble NaNO 3 or
KCl?
3. How does the line drawn for a
particular substance relate to the
saturation of a solution of that
substance?
4. How many grams of NH 4 Cl will
dissolve in 100 grams of 90°C
Water?
5. How many grams of NH 4 Cl will dissolve in 50 grams of 90°C water?
6. How many grams of KClO 3 will dissolve in 300 grams of 30°C water?
7. How would you make a saturated solution of KNO 3 at 60°C in 50 grams of
water?
8. If I asked you to make a saturated solution of KCl in 100 grams of water,
what other piece of information would you need before you could start?
9. You start with a saturated solution of NH 3 in 100 grams of water at 10°C.
How many grams of NH 3 gas would bubble out of the solution if you raise
the temperature to 80°C?
10. A saturated solution of KNO 3 in 400 grams of water at 50°C is cooled to
10°C. How much KNO 3 will come out of the solution as crystals?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








