Periodic Table Of The Elements
ADVERTISEMENT
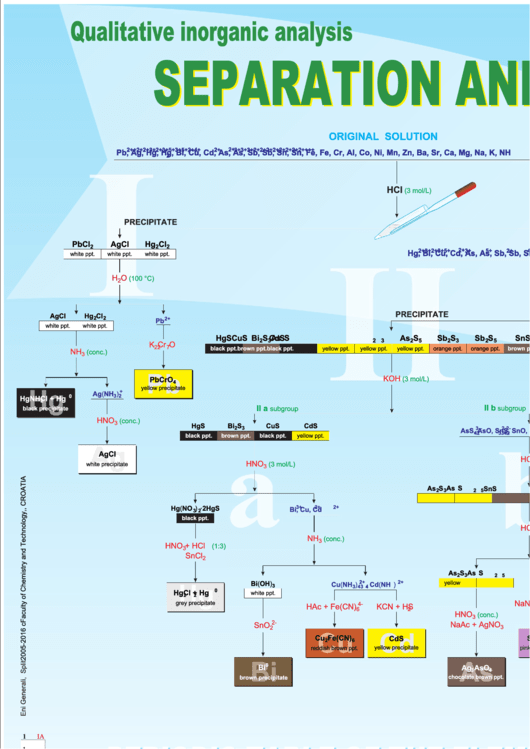
Qualitative inorganic analysis
SEPARATION AND IDENTIFICATION OF CATIONS
SEPARATION AND IDENTIFICATION OF CATIONS
O R I G I N A L S O L U T I O N
1540 °C
Nessler's reagent
HgO·Hg(NH )
HgO·Hg(NH )
2+
2+
+
+
2+
2+
2+
2+
3+
3+
2+
2+
2+
2+
3+
3+
5+
5+
3+
3+
5+
5+
2+
2+
4+
4+
2+
2+
3+
3+
3+
3+
3+
3+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
+
+
+
+
+
+
2 2
2 2
Pb , Ag , Hg , Hg , Bi , Cu , Cd , As , As , Sb , Sb , Sn , Sn , Fe , Fe , Cr , Al , Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
Pb , Ag , Hg , Hg , Bi , Cu , Cd , As , As , Sb , Sb , Sn , Sn , Fe , Fe , Cr , Al , Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
2
2
4
4
yellow to brown ppt.
Higher oxidation zone
1550 °C
(melting zone)
FLAME COLORING
FLAME COLORING
1560 °C
HCl
(3 mol/L)
Higher reduction zone
520 °C
Lower oxidation zone
PRECIPITATE
SOLUTION
1450 °C
Lower reduction zone
300 °C
PbCl
AgCl
Hg Cl
2
2
2
2+
2+
3+
3+
2+
2+
2+
2+
3+
3+
5+
5+
3+
3+
5+
5+
2+
2+
4+
4+
2+
2+
3+
3+
3+
3+
3+
3+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
+
+
+
+
+
+
Hg , Bi , Cu , Cd , As , As , Sb , Sb , Sn , Sn , Fe , Fe , Cr , Al , Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
Hg , Bi , Cu , Cd , As , As , Sb , Sb , Sn , Sn , Fe , Fe , Cr , Al , Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
white ppt.
white ppt.
white ppt.
4
4
Sodium
Potassium
H O
(100 °C)
H S
2
2
yellow
violet
(in acidic medium)
PRECIPITATE
SOLUTION
AgCl
Hg Cl
2
2
2+
Pb
white ppt.
white ppt.
2+
2+
3+
3+
3+
3+
3+
3+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
+
+
+
+
+
+
HgS
Bi S
CuS
CdS
As S
As S
Sb S
Sb S
SnS
SnS
Fe , Fe , Cr , Al , Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
Fe , Fe , Cr , Al , Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
2 3
2 3
2 5
2 3
2 5
2
4
4
K Cr O
2
2
7
black ppt.
brown ppt.
black ppt.
yellow ppt.
yellow ppt.
yellow ppt.
orange ppt.
orange ppt.
brown ppt.
yellow ppt.
NH
(conc.)
3
Calcium
Strontium
Barium
NH Cl + NH OH
brick red
crimson red
apple green
4
4
Pb
KOH
PbCrO
(3 mol/L)
4
yellow precipitate
+
Ag(NH )
0
3 2
HgNH Cl + Hg
PRECIPITATE
SOLUTION
2
II a
II b
subgroup
black precipitate
subgroup
HNO
(conc.)
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
2+
+
+
+
+
+
+
3
Fe(OH)
Cr(OH)
Al(OH)
Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
Co , Ni , Mn , Zn , Ba , Sr , Ca , Mg , Na , K , NH
HgS
Bi S
CuS
CdS
3
3
3
4
4
2 3
3-
-
2-
2-
3-
-
AsS , AsO , SnS , SnO , SbS , SbO
4
2
3
3
4
2
brown ppt.
grey-green p.
white ppt.
black ppt.
brown ppt.
black ppt.
yellow ppt.
H S
AgCl
2
HCl
(6 mol/L)
NaOH
+ H O
(6 mol/L)
(3 %)
(in basic medium)
HNO
white precipitate
(3 mol/L)
2
2
3
PRECIPITATE
SOLUTION
As S
As S
SnS
Sb S
Sb S
SnS
2 3
2 5
2
2 3
2 5
yellow ppt.
yellow ppt.
brown ppt.
yellow ppt.
orange ppt.
orange ppt.
2-
-
Hg(NO ) ·2HgS
3+
2+
2+
Fe(OH)
CrO , Al(OH)
Bi , Cu , Cd
3 2
3
4
4
CoS
NiS
MnS
ZnS
2+
2+
2+
2+
2+
2+
2+
2+
+
+
+
+
+
+
Ba , Sr , Ca , Mg , Na , K , NH
Ba , Sr , Ca , Mg , Na , K , NH
4
4
black ppt.
black ppt.
black ppt.
pink ppt.
white ppt.
brown ppt.
HCl
(conc.)
HAc + Pb(Ac)
NH Cl
2
4
NH
(conc.)
3
(NH ) PO
(0.5 mol/L)
HNO + HCl
HNO
(6 mol/L)
+ H O
(3 %)
4 2
4
(1:3)
3
2
2
HCl
(0.5 mol/L)
3
+ NH
(conc.)
SnCl
NH SCN
3
2
4
Cr
PbCrO
Al(OH)
4
3
As S
As S
PRECIPITATE
SOLUTION
white precipitate
2 3
2 5
3+
2+
yellow precipitate
Sb , Sn
2+
2+
Bi(OH)
yellow ppt.
yellow ppt.
Cu(NH )
, Cd(NH )
3
3 4
3 4
0
Hg Cl + Hg
white ppt.
2
2
2+
+
+
+
Fe(SCN)
CoS
NiS
2+
2+
Na , K ,
NH
Mn , Zn
Ba (PO )
Sr (PO )
Ca (PO )
MgNH PO
4
3
4 2
3
4 2
3
4 2
4
4
grey precipitate
NaNO +
rhodamine B
HCl + HgCl
4-
HAc + Fe(CN)
KCN + H S
2
2
blood red coloration
black ppt.
black ppt.
6
2
white ppt.
white ppt.
white ppt.
white ppt.
HNO
(conc.)
3
Zn-
Na Co(NO )
uranyl acetate
2-
NaAc + AgNO
SnO
3
2 6
NaOH
+ H O
2
3
(6 mol/L)
(3 %)
2
2
CH COOH
(conc.)
HNO
3
(conc.)
Cd
3
Cu Fe(CN)
CdS
Sb
4+
-rhodamine
Sn
K CrO
2
6
2
4
reddish brown ppt.
yellow precipitate
pinkish-red coloration
grey ppt.
Hg Cl
2
2
K
Na
uranyl acetate
K Co(NO )
-
3
2 6
2+
2+
pale yellow precipitate
yellow precipitate
0
Co , Ni
Bi
Ag AsO
-
3
4
MnO
Zn(OH) 3
2
2+
2+
2+
chocolate brown ppt.
Sr , Ca , Mg
brown precipitate
Ba
brown ppt.
BaCrO
4
yellow precipitate
NH SCN
NH
(3 mol/L)
4
3
NH
H S
(conc.)
+ amyl alcohol
+ dimethylglyoxime
3
2
HNO + NaBiO
3
3
Sr (PO )
Ca (PO )
MgNH PO
3
4 2
3
4 2
4
4
P E R I O D I C TA B L E O F T H E E L E M E N T S
P E R I O D I C TA B L E O F T H E E L E M E N T S
-
white ppt.
white ppt.
white ppt.
2-
MnO
1
IA
18
VIIIA
Co(SCN)
Ni
-dimethylglyoxime
ZnS
4
4
1
2
deep blue coloration
red precipitate
violet coloration
white precipitate
H
He
CH COOH
1
(conc.)
3
1.008
4.0026
(NH ) SO
2
IIA
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
4 2
4
5
6
7
8
9
10
3
4
Li
Be
B
C
N
O
F
Ne
2
Heating in the water bath
6.94
9.0122
10.81
12.011
14.007
15.999
18.998
20.180
Test tubes
11
12
13
14
15
16
17
18
2+
2+
Periodic table of the elements
Ca , Mg
Na
Mg
Al
Si
P
S
Cl
Ar
SrSO
3
4
VIIIB
white precipitate
Online calculators
22.990
24.305
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
8
9
10
11
IB
12
IIB
26.982
28.085
30.974
32.06
35.45
39.948
(NH ) C O
4 2 2
4
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
Downloads
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
4
39.098
40.078
44.956
47.867
50.942
51.996
54.938
55.845
58.933
58.693
63.546
65.38
69.723
72.64
74.922
78.971
79.904
83.798
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
2+
Mg
Rb
Sr
Y
Zr
Nb
Mo
Tc
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
5
CaC O
2
4
J. Meija et al, Atomic weights of the elements 2013, Pure Appl. Chem., 88, 265-291 (2016)
85.468
87.62
88.906
91.224
92.906
95.95
(98)
101.07
102.91
106.42
107.87
112.41
114.82
118.71
121.76
127.60
126.90
131.29
white precipitate
NaOH +
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
quinalizarine
Cs
Ba
La
Ce
Pr
Nd
Pm
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
Po
At
At
Rn
Rn
6
132.91
137.33
138.91
140.12
140.91
144.24
(145)
150.36
151.96
157.25
158.93
162.50
164.93
167.26
168.93
173.05
174.97
178.49
180.95
183.84
186.21
190.23
192.22
195.08
196.97
200.59
204.38
207.2
208.98
(209)
(210)
(222)
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
Mg
-quinalizarine
Fr
Fr
Ra
Ra
Ac
Ac
Th
Pa
U
Np
Np
Np
Np
Pu Am Cm Bk
Pu Am Cm Bk
Cf
Cf
Es
Es
Fm Md No
Fm Md No
Lr
Lr
Rf
Rf
Db
Db
Sg
Sg
Bh
Bh
Hs
Hs
Mt
Mt
Ds
Ds
Rg
Rg
Cn
Cn Uut
Uut
Fl Uup
Fl Uup Lv Uus Uuo
Lv
Uus
Uuo
7
Separate the clear liquid from the precipitate
blue coloration
(223)
(226)
(227)
232.04
231.04
238.03
(237)
(244)
(243)
(247)
(247)
(251)
(252)
(257)
(258)
(259)
(262)
(267)
(268)
(271)
(272)
(277)
(276)
(281)
(280)
(285)
(...)
(287)
(...)
(291)
(...)
(...)
Bunsen burner
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








