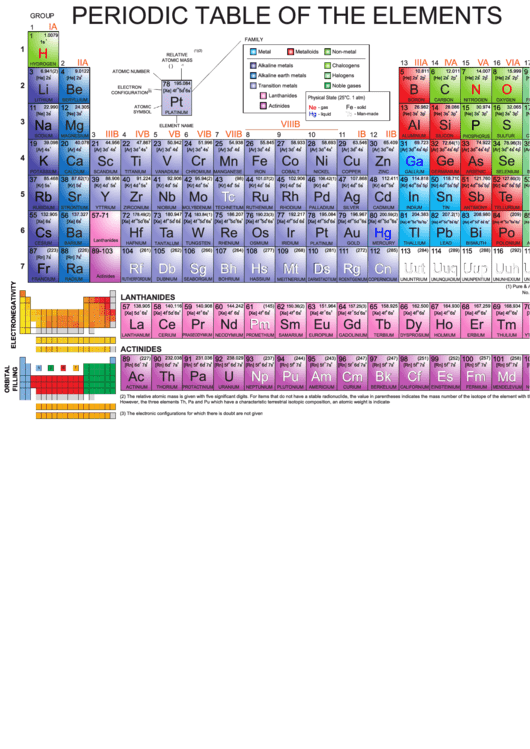
Periodic Table Of The Elements - Colorful
ADVERTISEMENT
PERIODIC TABLE OF THE ELEMENTS
GROUP
IA
VIIIA
1
18
1
2
1.0079
4.0026
FAMILY
1
2
1s
1s
1
H
He
1)(2)
Metal
Metalloids
Non-metal
(
RELATIVE
IIA
IIIA
IVA
VA
VIA
VIIA
ATOMIC MASS
13
14
15
16
17
2
HYDROGEN
HELIUM
Alkaline metals
Chalcogens
(g.mol )
-1
3
4
5
6
7
8
9
10
6.941(2)
9.0122
10.811
12.011
14.007
15.999
18.998
20.180
ATOMIC NUMBER
Alkaline earth metals
Halogens
[He] 2s
1
[He] 2s
2
[He] 2s 2p
2
1
[He] 2s 2p
2
2
[He] 2s 2p
2
3
[He] 2s 2p
2
4
[He] 2s 2p
2
5
[He] 2s 2p
2
6
Li
Be
78
B
C
N
O
F
Ne
2
195.084
Transition metals
Noble gases
ELECTRON
14
9
1
[Xe] 4f 5d 6s
(3)
CONFIGURATION
Lanthanides
Pt
Physical State (25°C. 1 atm)
LITHIUM
BERYLLIUM
BORON
CARBON
NITROGEN
OXYGEN
FLUORINE
NEON
Actinides
11
12
Ne
Fe
13
14
15
16
17
18
22.990
24.305
ATOMIC
26.982
28.086
30.974
32.065
35.453
39.948
- gas
- solid
SYMBOL
PLATINUM
Tc
1
2
Hg
2
1
2
2
2
3
2
4
2
5
2
6
[Ne] 3s
[Ne] 3s
- Man-made
[Ne] 3s 3p
[Ne] 3s 3p
[Ne] 3s 3p
[Ne] 3s 3p
[Ne] 3s 3p
[Ne] 3s 3p
- liquid
Na
Mg
AI
Si
P
S
Cl
Ar
3
VIIIB
ELEMENT NAME
IIIB
IVB
VB
VIB
VIIB
IB
IIB
3
4
5
6
7
8
9
10
11
12
SODIUM
MAGNESIUM
ALUMINUM
SILICON
PHOSPHORUS
SULFUR
CHLORINE
ARGON
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
39.098
40.078
44.956
47.867
50.942
51.996
54.938
55.845
58.933
58.693
63.546
65.409
69.723
72.64(1)
74.922
78.96(3)
79.904
83.798
1
2
1
2
2
2
3
2
5
1
5
2
6
2
7
2
8
2
10
1
10
2
10
2
1
10
2
2
10
2
3
10
2
4
10
2
5
10
2
6
[Ar] 4s
[Ar] 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s
[Ar] 3d 4s 4p
[Ar] 3d 4s 4p
[Ar] 3d 4s 4p
[Ar] 3d 4s 4p
[Ar] 3d 4s 4p
[Ar] 3d 4s 4p
K
Ca
Sc
Ti
V
Cr
Mn Fe
Co
Ni
Cu
Zn
Ga
Ge As
Se
Br
Kr
4
POTASSIUM
CALCIUM
SCANDIUM
TITANIUM
VANADIUM
CHROMIUM
MANGANESE
IRON
COBALT
NICKEL
COPPER
ZINC
GALLIUM
GERMANIUM
ARSENIC
SELENIUM
BROMINE
KRYPTON
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
85.468
87.62(1)
88.906
91.224
92.906
95.94(2)
(98)
101.07(2)
102.906
106.42(1)
107.868
112.411
114.818
118.710
121.760
127.60(3)
126.904
131.293
1
2
1
2
2
2
4
1
5
1
6
1
7
1
8
1
10
10
1
10
2
10
2
1
10
2
2
10
2
3
10
2
4
10
2
5
10
2
6
[Kr] 5s
[Kr] 5s
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d
[Kr] 4d 5s
[Kr] 4d 5s
[Kr] 4d 5s 5p
[Kr] 4d 5s 5p
[Kr] 4d 5s 5p
[Kr] 4d 5s 5p
[Kr] 4d 5s 5p
[Kr] 4d 5s 5p
Nb
Ru Rh Pd
Ag
I
Rb
Sr
Y
Zr
Mo
Tc
Cd
In
Sn
Sb
Te
Xe
5
RUBIDIUM
STRONTIUM
YTTRIUM
ZIRCONIUM
NIOBIUM
MOLYBDENUM
TECHNETIUM
RUTHENIUM
RHODIUM
PALLADIUM
SILVER
CADMIUM
INDIUM
ANTIMONY
TELLURIUM
IODINE
XENON
TIN
55
56
57-71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
132.905
137.327
178.49(2)
180.947
183.84(1)
186.207
190.23(3)
192.217
195.084
196.967
200.59(2)
204.383
207.2(1)
208.980
(209)
(210)
(222)
[Xe] 6s
1
2
[Xe] 4f 5d 6s
14
2
2
14
3
2
[Xe] 4f 5d 6s
14
4
2
[Xe] 4f 5d 6s
14
5
2
[Xe] 4f 5d 6s
14
6
2
[Xe] 4f 5d 6s
14
7
2
[Xe] 4f 5d 6s
14
9
1
14
10
1
14
10
2
[Xe] 6s
[Xe] 4f 5d 6s
[Xe] 4f 5d 6s
[Xe] 4f 5d 6s
14
10
2
1
14
10
2
2
14
10
2
3
14
10
2
4
14
10
2
5
14
10
2
6
[Xe] 4f 5d 6s 6p
[Xe] 4f 5d 6s 6p
[Xe] 4f 5d 6s 6p
[Xe] 4f 5d 6s 6p
[Xe] 4f 5d 6s 6p
[Xe] 4f 5d 6s 6p
Cs
Ba
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
6
Lanthanides
CESIUM
BARIUM
HAFNIUM
TANTALUM
TUNGSTEN
RHENIUM
OSMIUM
IRIDIUM
PLATINUM
GOLD
MERCURY
THALLIUM
LEAD
BISMUTH
POLONIUM
ASTATINE
RADON
87
88
89-103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
(223)
(226)
(261)
(262)
(266)
(264)
(277)
(268)
(281)
(272)
(285)
(284)
(289)
(288)
(292)
(294)
[Rn] 7s
1
[Rn] 7s
2
Fr
Ra
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Uut
Uuq
Uup
Uuh
Uus*
Uuo
7
Actinides
FRANCIUM
RADIUM
RUTHERFORDIUM
DUBNIUM
SEABORGIUM
BOHRIUM
HASSIUM
MEITNERIUM
DARMSTADTIUM ROENTGENIUM
COPERNICIUM
UNUNTRIUM
UNUNQUADIUM
UNUNPENTIUM
UNUNHEXIUM
UNUNOCTIUM
UNUNSEPTIUM
(1) Pure & Applied Chemistry, Vol. 78,
No. 11, pp. 2051–2066 (2006)
LANTHANIDES
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
138.905
140.116
140.908
144.242
(145)
150.36(2)
151.964
157.25(3)
158.925
162.500
164.930
167.259
168.934
173.04(3)
174.967
14
2
1
2
1
1
2
3
2
4
2
5
2
6
2
7
2
7
1
2
9
2
10
2
11
2
12
2
13
2
14
1
2
[Xe] 5d 6s
[Xe] 4f 5d 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 5d 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 6s
[Xe] 4f 5d 6s
Nd
Pm
Tb
Lu
La
Ce
Pr
Sm
Eu
Gd
Dy
Ho
Er
Tm
Yb
LANTHANUM
CERIUM
PRASEODYMIUM
NEODYMIUM
PROMETHIUM
SAMARIUM
EUROPIUM
GADOLINIUM
TERBIUM
DYSPROSIUM
HOLMIUM
ERBIUM
THULIUM
YTTERBIUM
LUTETIUM
ACTINIDES
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
(227)
232.038
231.036
238.029
(237)
(244)
(243)
(247)
(247)
(251)
(252)
(257)
(258)
(259)
(262)
1
2
2
2
2
1
2
3
1
2
4
1
2
6
2
7
2
7
1
2
9
2
10
2
11
2
12
2
13
2
14
2
[Rn] 6d 7s
[Rn] 6d 7s
[Rn] 5f 6d 7s
[Rn] 5f 6d 7s
[Rn] 5f 6d 7s
[Rn] 5f 7s
[Rn] 5f 7s
[Rn] 5f 6d 7s
[Rn] 5f 7s
[Rn] 5f 7s
[Rn] 5f 7s
[Rn] 5f 7s
[Rn] 5f 7s
[Rn] 5f 7s
s
p
d
f
Np
Pu Am
Bk
Cf
Es
Fm
No
Lr
Ac
Th
Pa
U
Cm
Md
ACTINIUM
THORIUM
PROTACTINIUM
URANIUM
NEPTUNIUM PLUTONIUM
AMERICIUM
CURIUM
BERKELIUM
CALIFORNIUM
EINSTEINIUM
FERMIUM
MENDELEVIUM
NOBELIUM
LAWRENCIUM
(2) The relative atomic mass is given with five significant digits. For items that do not have a stable radionuclide, the value in parentheses indicates the mass number of the isotope of the element with the longest half-life.
However, the three elements Th, Pa and Pu which have a characteristic terrestrial isotopic composition, an atomic weight is indicated.
(3) The electronic configurations for which there is doubt are not given.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








