Effective Nuclear Charge (Zeff) And Atomic Radius
ADVERTISEMENT
Effective Nuclear Charge (Z
) and Atomic Radius
eff
Chemical properties of an element are largely determined by their valence electrons. Elements with similarly filled valence
1
shells have similar properties. For example, all the alkali metals have a electron configuration ns
. All the halogens have an
2
5
electron configuration ending with ns
np
. What is a common characteristic shared by all the halogens?
What is a common characteristic shared by the noble gasses? What is their generic configuration?
We now explore trends in chemical properties that are related to electron configuration and valence shells.
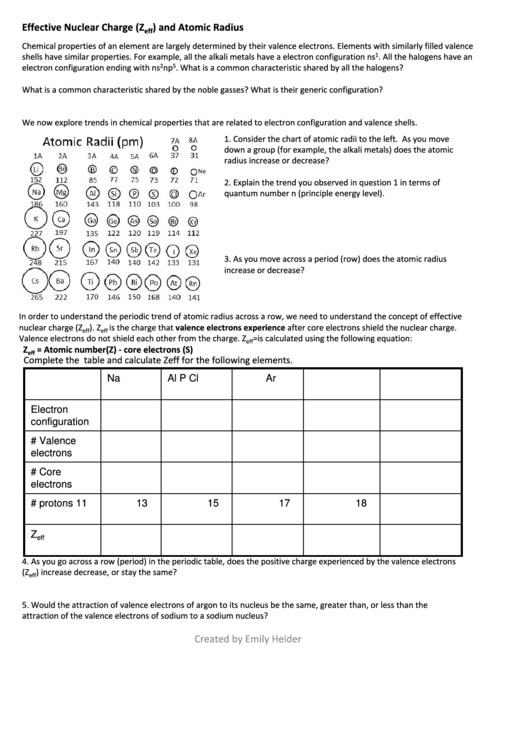
1. Consider the chart of atomic radii to the left. As you move
down a group (for example, the alkali metals) does the atomic
radius increase or decrease?
2. Explain the trend you observed in question 1 in terms of
quantum number n (principle energy level).
3. As you move across a period (row) does the atomic radius
increase or decrease?
In order to understand the periodic trend of atomic radius across a row, we need to understand the concept of effective
nuclear charge (Z
). Z
is the charge that valence electrons experience after core electrons shield the nuclear charge.
eff
eff
Valence electrons do not shield each other from the charge. Z
=is calculated using the following equation:
eff
Z
= Atomic number(Z) - core electrons (S)
eff
Complete the table and calculate Zeff for the following elements.
Na
Al
P
Cl
Ar
Electron
configuration
# Valence
electrons
# Core
electrons
# protons
11
13
15
17
18
Z
eff
4. As you go across a row (period) in the periodic table, does the positive charge experienced by the valence electrons
(Z
) increase decrease, or stay the same?
eff
5. Would the attraction of valence electrons of argon to its nucleus be the same, greater than, or less than the
attraction of the valence electrons of sodium to a sodium nucleus?
Created by Emily Heider
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








