Molecular Modeling
ADVERTISEMENT
Central
Bonding System
Electronic
Molecular
Valence Bond
Polar or
Species
Name
Atom
(A
B
U
)
Geometry
Geometry
Hybridization
Non polar
x
y
z
PO 4 3-
3
Phosphate ion
P
AB
Tetrahedral
Tetrahedral
sp
Polar
4
SO 4 2-
3
Sulfate ion
S
AB
Tetrahedral
Tetrahedral
sp
Polar
4
CO 3 2-
2
Carbonate ion
C
AB
Trigonal planar
Trigonal planar
sp
Polar
3
NO 3 -
2
Nitrate ion
N
AB
Trigonal planar
Trigonal planar
sp
Polar
3
ClO 4 -
3
Perchlorate ion
Cl
AB
Tetrahedral
Tetrahedral
sp
Polar
4
3
NH 3
Ammonia
N
AB
U
Tetrahedral
Trigonal pyramidal
sp
Polar
3
CO 2
Carbon dioxide
C
AB
Linear
Linear
sp
Non polar
2
3
H 2 O
Dihydrogen monoxide
O
AB
U
Tetrahedral
Angular
sp
Polar
2
2
Ne
Neon
Ne
NA
NA
NA
NA
NA
Cl -
Chloride ion
Cl
NA
NA
NA
NA
Polar
1
1
1
1
N
: ABU
N
: Linear
N
: Linear
N
: sp
N 2
Nitrogen
N, N
Non polar
2
2
2
2
N
: ABU
N
: Linear
N
: Linear
N
: sp
3
HCl
Hydrogen chloride
Cl
ABU
Tetrahedral
Linear
sp
Polar
3
3
CH 4
Methane
C
AB
Tetrahedral
Tetrahedral
sp
Non polar
4
CCl 4
3
Carbon tetrachloride
C
AB
Tetrahedral
Tetrahedral
sp
Non polar
4
2
N: AB
N: trigonal planar
N: trigonal planar
N: sp
HNO 3
3
Nitric acid
N, O
Polar
3
O: AB
U
O: tetrahedral
O: Angular
O: sp
2
2
3
S: AB
S: tetrahedral
S: tetrahedral
S: sp
4
H 2 SO 4
1
1
1
1
3
Sulfuric acid
S, O, O
O
: AB
U
O
: tetrahedral
O
: angular
O
: sp
Polar
2
2
2
2
2
2
3
O
: AB
U
O
: tetrahedral
O
: angular
O
: sp
2
2
1
1
1
1
2
C
: AB
C
: trigonal planar
C
: trigonal planar
C
: sp
CH 2 CH 2
3
Ethylene
C, C
Non polar
2
2
2
2
2
C
: AB
C
: trigonal planar
C
: trigonal planar
C
: sp
3
1
1
1
1
3
C
: AB
C
: tetrahedral
C
: tetrahedral
C
: sp
CH 3 CH 3
4
Ethane
C, C
Non polar
2
2
2
2
3
C
: AB
C
: tetrahedral
C
: tetrahedral
C
: sp
4
2
O 3
Ozone
O
AB
U
Trigonal planar
Angular
sp
Polar
2
SF 4
3
Sulfur tetrafluoride
S
AB
U
Trigonal bipyramidal
See saw
sp
d
Polar
4
3
PF 5
Phosphorus pentafluoride
P
AB
Trigonal bipyramidal
Trigonal bipyramidal
sp
d
Polar
5
I 3 -
3
Triiodide ion
I
AB
U
Trigonal bipyramidal
Linear
sp
d
Polar
2
3
PF 6 -
3
2
Phosphorous hexafluoride ion
P
AB
Octahedral
Octahedral
sp
d
Polar
6
BrF 5
3
2
Bromine pentafluoride
Br
AB
U
Octahedral
Square pyramidal
sp
d
Polar
5
IF 4 -
3
2
Iodine tetrafluoride ion
I
AB
U
Octahedral
Square planar
sp
d
Polar
4
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2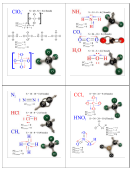 3
3 4
4








