Chemistry: Safety Symbols, Matter & Atomic Review
ADVERTISEMENT
SNC2P
CHEMISTRY: SAFETY SYMBOLS, MATTER & ATOMIC REVIEW
QUIZ#1
1.
What do the following acronym s stand for?
{2}
(a) WHMIS
(b) HHPS
2.
Why is it necessary to have a system like WHMIS in the workplace. How does it benefit the employee or employer?
{2}
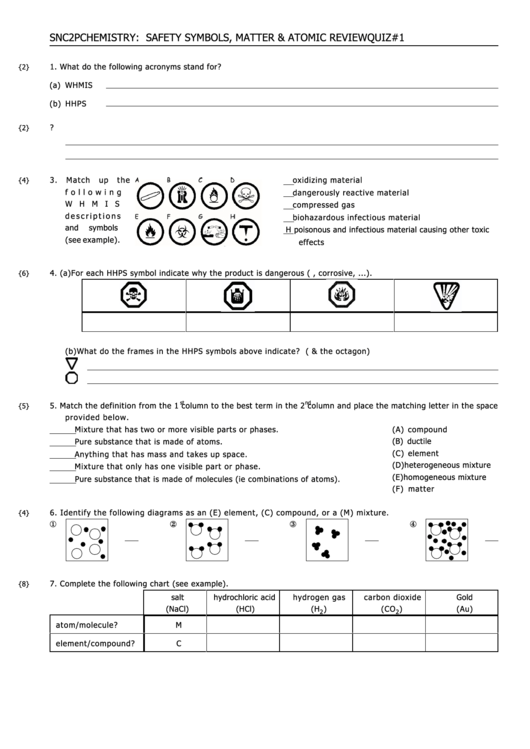
3.
M a tch up the
oxidizing material
{4}
f o l l o w i n g
dangerously reactive material
W H M I S
compressed gas
d e s c r i p t i o n s
biohazardous infectious material
and
symbols
H poisonous and infectious material causing other toxic
(see example).
effects
4.
(a) For each HHPS symbol indicate why the product is dangerous (i.e. flammable, corrosive, ...).
{6}
(b) What do the frames in the HHPS symbols above indicate? (i.e. the triangle & the octagon)
st
nd
5.
Match the definition from the 1 colum n to the best term in the 2
colum n and place the matching letter in the space
{5}
provided below.
Mixture that has two or more visible parts or phases.
(A) compound
(B) ductile
Pure substance that is made of atoms.
(C) element
Anything that has mass and takes up space.
(D) heterogeneous mixture
Mixture that only has one visible part or phase.
(E) homogeneous mixture
Pure substance that is made of molecules (ie combinations of atom s).
(F) matter
6.
Identify the following diagrams as an (E) element, (C) compound, or a (M) mixture.
{4}
7.
Com plete the following chart (see example).
{8}
salt
hydrochloric acid
hydrogen gas
carbon dioxide
Gold
(NaCl)
(HCl)
(H )
(CO )
(Au)
2
2
atom/molecule?
M
element/compound?
C
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








