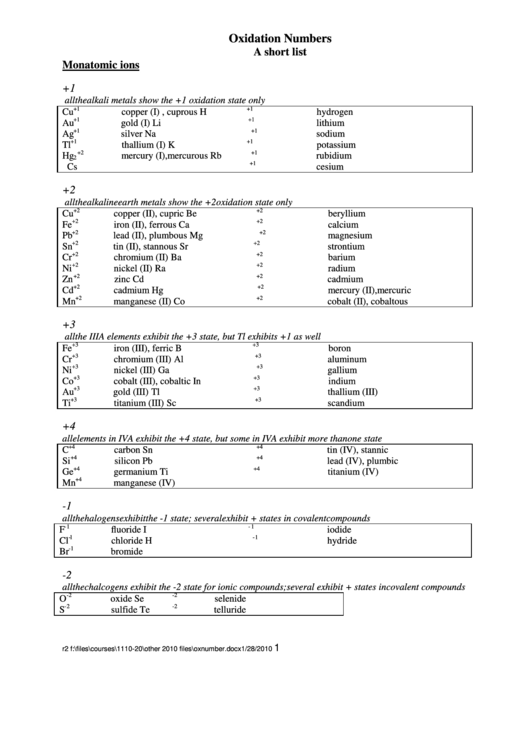
Oxidation Numbers
ADVERTISEMENT
Oxidation Numbers
A short list
Monatomic ions
+1
all the alkali metals show the +1 oxidation state only
+1
+1
Cu
copper (I) , cuprous
H
hydrogen
+1
+1
Au
gold (I)
Li
lithium
+1
+1
Ag
silver
Na
sodium
+1
+1
Tl
thallium (I)
K
potassium
+2
+1
Hg
mercury (I), mercurous
Rb
rubidium
2
+1
Cs
cesium
+2
all the alkaline earth metals show the +2 oxidation state only
+2
+2
Cu
copper (II), cupric
Be
beryllium
+2
+2
Fe
iron (II), ferrous
Ca
calcium
+2
+2
Pb
lead (II), plumbous
Mg
magnesium
+2
+2
Sn
tin (II), stannous
Sr
strontium
+2
+2
Cr
chromium (II)
Ba
barium
+2
+2
Ni
nickel (II)
Ra
radium
+2
+2
Zn
zinc
Cd
cadmium
+2
+2
Cd
cadmium
Hg
mercury (II), mercuric
+2
+2
Mn
manganese (II)
Co
cobalt (II), cobaltous
+3
all the IIIA elements exhibit the +3 state, but Tl exhibits +1 as well
+3
+3
Fe
iron (III), ferric
B
boron
+3
+3
Cr
chromium (III)
Al
aluminum
+3
+3
Ni
nickel (III)
Ga
gallium
+3
+3
Co
cobalt (III), cobaltic
In
indium
+3
+3
Au
gold (III)
Tl
thallium (III)
+3
+3
Ti
titanium (III)
Sc
scandium
+4
all elements in IVA exhibit the +4 state, but some in IVA exhibit more than one state
+4
+4
C
carbon
Sn
tin (IV), stannic
+4
+4
Si
silicon
Pb
lead (IV), plumbic
+4
+4
Ge
germanium
Ti
titanium (IV)
+4
Mn
manganese (IV)
-1
all the halogens exhibit the -1 state; several exhibit + states in covalent compounds
-1
-1
F
fluoride
I
iodide
-1
-1
Cl
chloride
H
hydride
-1
Br
bromide
-2
all the chalcogens exhibit the -2 state for ionic compounds; several exhibit + states in covalent compounds
-2
-2
O
oxide
Se
selenide
-2
-2
S
sulfide
Te
telluride
1
r2 f:\files\courses\1110-20\other 2010 files\oxnumber.docx1/28/2010
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








