Mechanism Summary For As Chemistry
ADVERTISEMENT
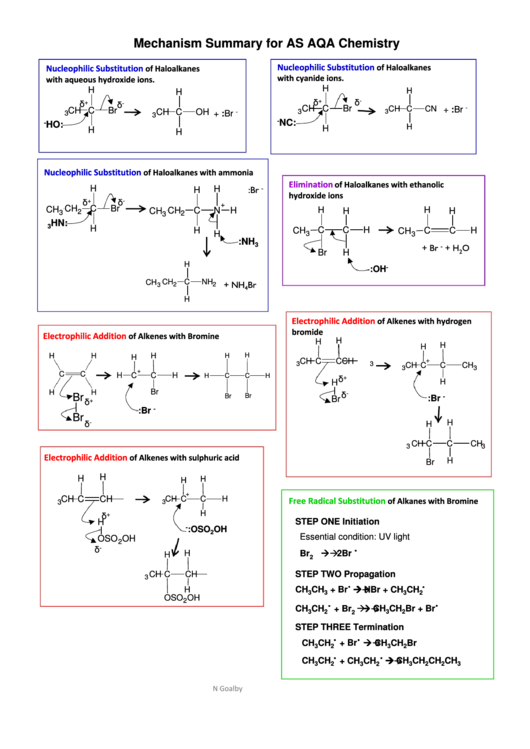
Mechanism Summary for AS AQA Chemistry
Nucleophilic Substitution
of Haloalkanes
Nucleophilic Substitution
of Haloalkanes
with cyanide ions.
with aqueous hydroxide ions.
H
H
H
H
+
-
δ
δ
+
δ
-
δ
H
C
C
Br
-
H
C
C
CN
+ :Br
H
C
C
Br
H
C
C
OH
3
3
-
+ :Br
3
3
-
NC:
-
HO:
H
H
H
H
Nucleophilic Substitution
of Haloalkanes with ammonia
Elimination
of Haloalkanes with ethanolic
H
H
-
H
:Br
hydroxide ions
+
-
δ
δ
+
CH
C
Br
CH
CH
C
N
H
H
H
CH
H
H
2
3
2
3
HN:
3
H
H
C
C
H
CH
C
C
H
CH
H
3
3
:NH
3
-
+ Br
+ H
O
Br
H
2
H
-
:OH
CH
C
NH
CH
+ NH
Br
2
2
3
4
H
Electrophilic Addition
of Alkenes with hydrogen
bromide
Electrophilic Addition
of Alkenes with Bromine
H
H
H
H
H
H
H
H
H
H
H
C
C
C
CH
+
3
3
H
C
C
C
CH
3
3
+
C
C
H
C
C
H
H
C
C
H
+
δ
H
H
Br
H
H
-
δ
Br
Br
Br
-
:Br
Br
+
δ
-
:Br
Br
H
-
δ
H
H
C
C
C
CH
3
3
Electrophilic Addition
of Alkenes with sulphuric acid
H
Br
H
H
H
H
+
H
C
C
C
H
H
C
C
C
H
Free Radical Substitution
of Alkanes with Bromine
3
3
H
+
δ
STEP ONE Initiation
H
-
:OSO
OH
2
Essential condition: UV light
OSO
OH
2
.
-
δ
H
Br
2Br
H
2
STEP TWO Propagation
H
C
C
C
H
3
.
.
H
CH
CH
+ Br
HBr + CH
CH
3
3
3
2
OSO
OH
2
.
.
CH
CH
+ Br
CH
CH
Br + Br
3
2
3
2
2
STEP THREE Termination
.
.
CH
CH
+ Br
CH
CH
Br
3
2
3
2
.
.
CH
CH
+ CH
CH
CH
CH
CH
CH
3
2
3
2
3
2
2
3
N Goalby
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2