Chemistry Data Sheet And Periodic Table Of The Elements
ADVERTISEMENT
HIGHER SCHOOL CERTIFIC ATE EXAMINATION
Chemistry
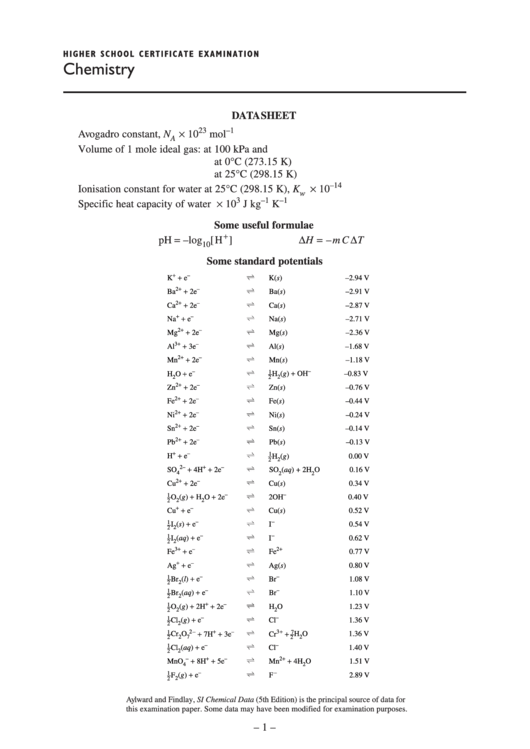
DATA SHEET
.................................................................. 6.022 × 10
23
–1
Avogadro constant, N
mol
A
Volume of 1 mole ideal gas: at 100 kPa and
at 0°C (273.15 K) ...................... 22.71 L
at 25°C (298.15 K) .................... 24.79 L
................. 1.0 × 10
–14
Ionisation constant for water at 25°C (298.15 K), K
w
Specific heat capacity of water ..................................................... 4.18 × 10
3
–1
–1
J kg
K
Some useful formulae
+
pH = –log
∆H = –m C ∆T
[H
]
10
Some standard potentials
+
+ e
–
K
K(s)
–2.94 V
2+
+ 2e
–
Ba
Ba(s)
–2.91 V
2+
+ 2e
–
Ca
Ca(s)
–2.87 V
+
+ e
–
Na
Na(s)
–2.71 V
2+
+ 2e
–
Mg
Mg(s)
–2.36 V
3+
+ 3e
–
Al
Al(s)
–1.68 V
2+
+ 2e
–
Mn
Mn(s)
–1.18 V
O + e
–
(g) + OH
–
1
H
–
H
–0.83 V
2
2
2
2+
+ 2e
–
Zn
Zn(s)
–0.76 V
2+
+ 2e
–
Fe
Fe(s)
–0.44 V
+ 2e
2+
–
Ni
Ni(s)
–0.24 V
2+
+ 2e
–
Sn
Sn(s)
–0.14 V
2+
+ 2e
–
Pb
Pb(s)
–0.13 V
+
+ e
–
1
H
–
H
(g)
0.00 V
2
2
+
+ 4H
+ 2e
2–
–
SO
SO
(aq) + 2H
O
0.16 V
4
2
2
2+
+ 2e
–
Cu
Cu(s)
0.34 V
(g) + H
O + 2e
–
–
1
–
O
2OH
0.40 V
2
2
2
+
+ e
–
Cu
Cu(s)
0.52 V
(s) + e
–
–
1
–
I
I
0.54 V
2
2
(aq) + e
–
–
1
–
I
I
0.62 V
2
2
3+
+ e
–
2+
Fe
Fe
0.77 V
+
+ e
–
Ag
Ag(s)
0.80 V
(l) + e
–
–
1
–
Br
Br
1.08 V
2
2
(aq) + e
–
–
1
–
Br
Br
1.10 V
2
2
+
(g) + 2H
+ 2e
–
1
–
O
H
O
1.23 V
2
2
2
(g) + e
–
–
1
–
Cl
Cl
1.36 V
2
2
+
2–
+ 7H
+ 3e
–
3+
+
1
7
–
Cr
O
Cr
–
H
O
1.36 V
2
7
2
2
2
(aq) + e
–
–
1
–
Cl
Cl
1.40 V
2
2
+
–
+ 8H
+ 5e
–
2+
+ 4H
MnO
Mn
O
1.51 V
4
2
(g) + e
–
–
1
–
F
F
2.89 V
2
2
Aylward and Findlay, SI Chemical Data (5th Edition) is the principal source of data for
this examination paper. Some data may have been modified for examination purposes.
– 1 –
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








