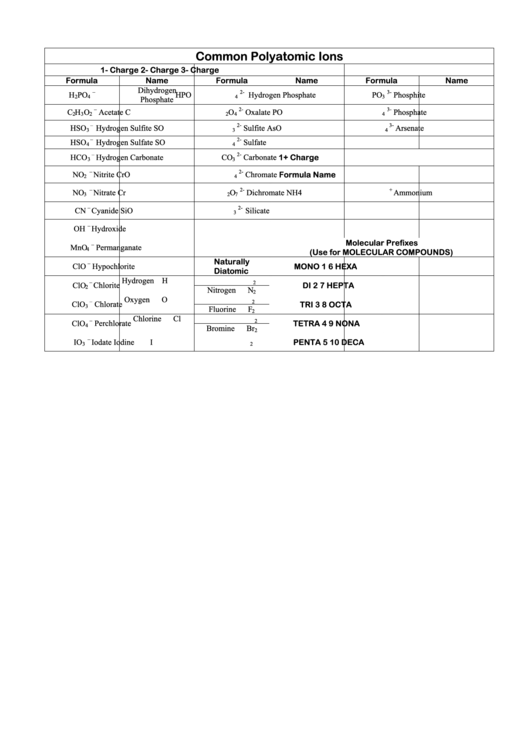
Common Polyatomic Ions Chart
ADVERTISEMENT
Common Polyatomic Ions
1- Charge
2- Charge
3- Charge
Formula
Name
Formula
Name
Formula
Name
Dihydrogen
–
2-
3-
H
PO
HPO
Hydrogen Phosphate
PO
Phosphite
2
4
4
3
Phosphate
–
2-
3-
C
H
O
Acetate
C
O
Oxalate
PO
Phosphate
2
3
2
2
4
4
–
2-
3-
HSO
Hydrogen Sulfite
SO
Sulfite
AsO
Arsenate
3
3
4
–
2-
HSO
Hydrogen Sulfate
SO
Sulfate
4
4
–
2-
HCO
Hydrogen Carbonate
CO
Carbonate
1+ Charge
3
3
–
2-
NO
Nitrite
CrO
Chromate
Formula
Name
2
4
–
2-
+
NO
Nitrate
Cr
O
Dichromate
NH4
Ammonium
3
2
7
–
2-
CN
Cyanide
SiO
Silicate
3
–
OH
Hydroxide
Molecular Prefixes
–
MnO
Permanganate
4
(Use for MOLECULAR COMPOUNDS)
Naturally
–
ClO
Hypochlorite
MONO
1
6
HEXA
Diatomic
Hydrogen H
–
2
ClO
Chlorite
DI
2
7
HEPTA
2
Nitrogen
N
2
Oxygen
O
–
2
ClO
Chlorate
TRI
3
8
OCTA
3
Fluorine
F
2
Chlorine
Cl
–
2
ClO
Perchlorate
TETRA
4
9
NONA
4
Bromine
Br
2
–
IO
Iodate
Iodine
I
PENTA
5
10
DECA
3
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








