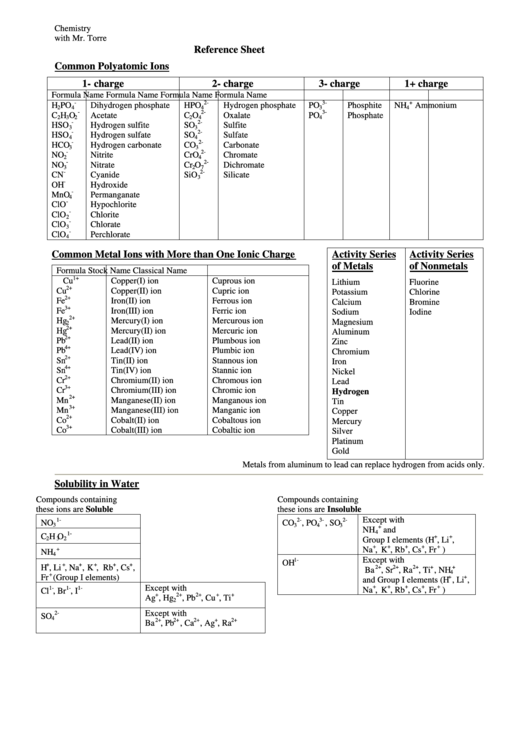
Common Polyatomic Ions Reference Sheet
ADVERTISEMENT
Chemistry
with Mr. Torre
Reference Sheet
Common Polyatomic Ions
1- charge
2- charge
3- charge
1+ charge
Formula
Name
Formula
Name
Formula
Name
Formula
Name
-
2-
3-
+
H
PO
Dihydrogen phosphate
HPO
Hydrogen phosphate
PO
Phosphite
NH
Ammonium
2
4
4
3
4
-
2-
3-
C
H
O
Acetate
C
O
Oxalate
PO
Phosphate
2
3
2
2
4
4
-
2-
HSO
Hydrogen sulfite
SO
Sulfite
3
3
-
2-
HSO
Hydrogen sulfate
SO
Sulfate
4
4
-
2-
HCO
Hydrogen carbonate
CO
Carbonate
3
3
-
2-
NO
Nitrite
CrO
Chromate
2
4
-
2-
NO
Nitrate
Cr
O
Dichromate
3
2
7
-
2-
CN
Cyanide
SiO
Silicate
3
-
OH
Hydroxide
-
MnO
Permanganate
4
-
ClO
Hypochlorite
-
ClO
Chlorite
2
-
ClO
Chlorate
3
-
ClO
Perchlorate
4
Common Metal Ions with More than One Ionic Charge
Activity Series
Activity Series
of Metals
of Nonmetals
Formula
Stock Name
Classical Name
1+
Cu
Copper(I) ion
Cuprous ion
Lithium
Fluorine
2+
Cu
Copper(II) ion
Cupric ion
Potassium
Chlorine
2+
Fe
Iron(II) ion
Ferrous ion
Calcium
Bromine
3+
Fe
Iron(III) ion
Ferric ion
Sodium
Iodine
2+
Hg
Mercury(I) ion
Mercurous ion
Magnesium
2
2+
Hg
Mercury(II) ion
Mercuric ion
Aluminum
2+
Pb
Lead(II) ion
Plumbous ion
Zinc
4+
Pb
Lead(IV) ion
Plumbic ion
Chromium
2+
Sn
Tin(II) ion
Stannous ion
Iron
4+
Sn
Tin(IV) ion
Stannic ion
Nickel
2+
Cr
Chromium(II) ion
Chromous ion
Lead
3+
Cr
Chromium(III) ion
Chromic ion
Hydrogen
2+
Mn
Manganese(II) ion
Manganous ion
Tin
3+
Mn
Manganese(III) ion
Manganic ion
Copper
2+
Co
Cobalt(II) ion
Cobaltous ion
Mercury
3+
Co
Cobalt(III) ion
Cobaltic ion
Silver
Platinum
Gold
Metals from aluminum to lead can replace hydrogen from acids only.
Solubility in Water
Compounds containing
Compounds containing
these ions are Soluble
these ions are Insoluble
Except with
1-
2-
3-
2-
NO
CO
, PO
, SO
3
3
4
3
+
NH
and
4
1-
C
H
O
+
+
2
3
2
Group I elements (H
, Li
,
+
+
+
+
+
Na
, K
, Rb
, Cs
, Fr
)
+
NH
4
Except with
1-
OH
+
+
+
+
+
+
H
, Li
, Na
, K
, Rb
, Cs
,
2+
2+
2+
+
+
Ba
, Sr
, Ra
, Ti
, NH
4
+
Fr
(Group I elements)
+
+
and Group I elements (H
, Li
,
Except with
+
+
+
+
+
1-
1-
1-
Na
, K
, Rb
, Cs
, Fr
)
Cl
, Br
, I
+
2+
2+
+
+
Ag
, Hg
, Pb
, Cu
, Ti
2
Except with
2-
SO
4
2+
2+
2+
+
2+
Ba
, Pb
, Ca
, Ag
, Ra
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








