Energy Profiles Worksheet
ADVERTISEMENT
C h e m g u i d e – q u e s t i o n s
ENERGY PROFILES
1. A simple reaction can involve a mechanism which either goes via a transition state or an
intermediate. This question looks at transition states.
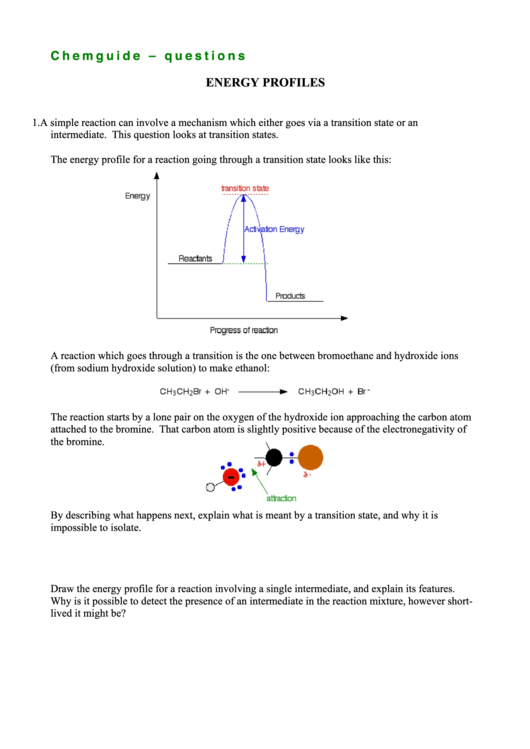
The energy profile for a reaction going through a transition state looks like this:
A reaction which goes through a transition is the one between bromoethane and hydroxide ions
(from sodium hydroxide solution) to make ethanol:
The reaction starts by a lone pair on the oxygen of the hydroxide ion approaching the carbon atom
attached to the bromine. That carbon atom is slightly positive because of the electronegativity of
the bromine.
By describing what happens next, explain what is meant by a transition state, and why it is
impossible to isolate.
2. This question is about energy profiles where there is an intermediate.
Draw the energy profile for a reaction involving a single intermediate, and explain its features.
Why is it possible to detect the presence of an intermediate in the reaction mixture, however short-
lived it might be?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1