Extraction Of Metals
ADVERTISEMENT
4/26/2010
Learning Objectives
Extraction of Metals
• Describe the ease of obtaining metals
from their ores by relating the elements to
their position in the reactivity series
• Describe the essential reactions in the
extraction of iron from Haematite using the
blast furnace.
Recap
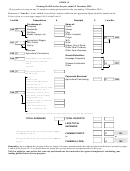
Why are metals extracted?
• Reactivity series
• Most metals are found in the Earth’s crust
combined with other elements in rocks
• Reaction of metals with water/steam
known as ores.
• Reaction of metals with dilute HCl
• An ore is a compound of the metal mixed
with large amounts of earth and rock.
• Metals need to be extracted from ores
before they can be turned into useful
products.
What methods are used to extract metals?
Question
• Metals that are found in the ground as
How do mining companies decide
uncombined elements do not require
which method to use?
further extraction.
– E.g. Gold
• Obtaining metals from their ores requires
chemical reactions:
1. Reducing the ore to the metal using carbon
2. Using electrolysis method
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4