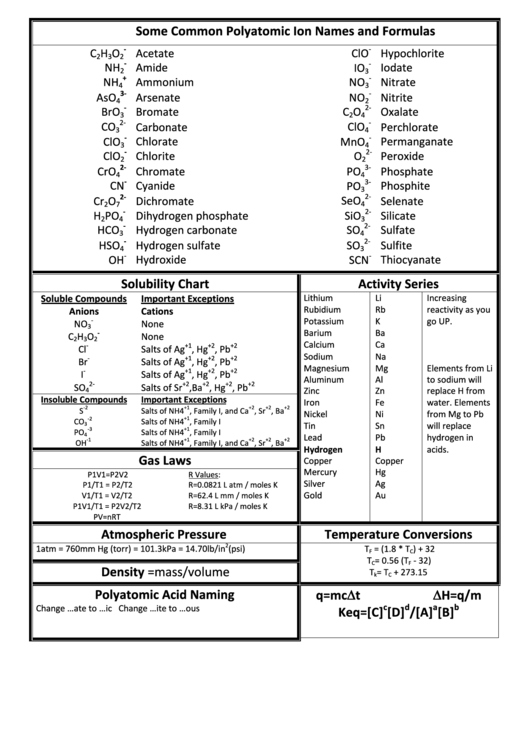
Some Common Polyatomic Ion Names And Formulas Solubility Chart
ADVERTISEMENT
Some Common Polyatomic Ion Names and Formulas
-
-
C
H
O
Acetate
ClO
Hypochlorite
2
3
2
-
-
NH
Amide
IO
Iodate
2
3
+
-
NH
Ammonium
NO
Nitrate
4
3
3-
-
AsO
Arsenate
NO
Nitrite
4
2
-
2-
BrO
Bromate
C
O
Oxalate
3
2
4
2-
-
CO
Carbonate
ClO
Perchlorate
3
4
-
-
ClO
Chlorate
MnO
Permanganate
3
4
-
2-
ClO
Chlorite
O
Peroxide
2
2
2-
3-
CrO
Chromate
PO
Phosphate
4
4
-
3-
CN
Cyanide
PO
Phosphite
3
2-
2-
Cr
O
Dichromate
SeO
Selenate
2
7
4
-
2-
H
PO
Dihydrogen phosphate
SiO
Silicate
2
4
3
-
2-
HCO
Hydrogen carbonate
SO
Sulfate
3
4
-
2-
HSO
Hydrogen sulfate
SO
Sulfite
4
3
-
-
OH
Hydroxide
SCN
Thiocyanate
Solubility Chart
Activity Series
Lithium
Li
Increasing
Soluble Compounds
Important Exceptions
Rubidium
Rb
reactivity as you
Anions
Cations
-
Potassium
K
go UP.
NO
None
3
Barium
Ba
-
C
H
O
None
2
3
2
Calcium
Ca
-
+1
+2
+2
Cl
Salts of Ag
, Hg
, Pb
Sodium
Na
-
+1
+2
+2
Br
Salts of Ag
, Hg
, Pb
Magnesium
Mg
Elements from Li
-
+1
+2
+2
I
Salts of Ag
, Hg
, Pb
Aluminum
Al
to sodium will
2-
+2
+2
+2
+2
SO
Salts of Sr
,Ba
, Hg
, Pb
4
Zinc
Zn
replace H from
Insoluble Compounds
Important Exceptions
Iron
Fe
water. Elements
-2
+1
+2
+2
+2
S
Salts of NH4
, Family I, and Ca
, Sr
, Ba
Nickel
Ni
from Mg to Pb
-2
+1
CO
Salts of NH4
, Family I
3
Tin
Sn
will replace
-3
+1
PO
Salts of NH4
, Family I
4
Lead
Pb
hydrogen in
-1
+1
+2
+2
+2
OH
Salts of NH4
, Family I, and Ca
, Sr
, Ba
Hydrogen
H
acids.
Gas Laws
Copper
Copper
Mercury
Hg
P1V1=P2V2
R Values:
Silver
Ag
P1/T1 = P2/T2
R=0.0821 L atm / moles K
Gold
Au
V1/T1 = V2/T2
R=62.4 L mm / moles K
P1V1/T1 = P2V2/T2
R=8.31 L kPa / moles K
PV=nRT
Atmospheric Pressure
Temperature Conversions
2
1atm = 760mm Hg (torr) = 101.3kPa = 14.70lb/in
(psi)
T
= (1.8 * T
) + 32
F
C
T
= 0.56 (T
- 32)
C
F
Density =mass/volume
T
= T
+ 273.15
k
C
H=q/m
Polyatomic Acid Naming
q=mct
c
d
a
b
Change …ate to …ic Change …ite to …ous
Keq=[C]
[D]
/[A]
[B]
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








