Periodic Table Of The Elements Based On The Electronic Structure Of Atoms Albert Tarantola (1975) Page 2
ADVERTISEMENT
1
2
PERIODIC TABLE OF THE ELEMENTS
H
He
3
4
Based on the electronic structure of atoms
Li
Be
Albert Tarantola (1975)
5
6
7
8
9
10
11
12
B
C
N
O
F
Ne
Na
Mg
13
14
15
16
17
18
19
20
Al
Si
P
S
Cl
A
K
Ca
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
Ba
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
La
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
Fr
Ra
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
Ac
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
Rf
Db
Sg
Bh
Hs
Mt
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
Xx
138
139 140
141 142
143 144
145 146
147 148
149 150
151 152
153 154
155 156
157 158
159 160
161 162
163 164
165 166
167 168
169 170
1s
2s
3s
2p
4s
3p
5s
3d
4p
6s
4d
5p
4f
5d
6p
7s
5f
8s
6d
7p
This table results from a simple filling of the natural classification of the energy levels of the atoms.
It allows a direct reading of the electronic structure.
2
2
6
2
4
As an example, for element (S,16) we have
1s
2s
2p
3s
3p
.
As some energy levels are quite close, some elements may have one or two electrons "misplaced".
These exceptional elements are: Cr, Cu, Nb, Mo, Ru, Rh, Pd, Ag, La, Gd, Pt, Au, Ac, Th, Pa, U and Cm.
Figure 1: The Table of the Elements and the filling of the atomic orbitals.
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1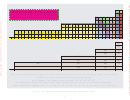 2
2 3
3








