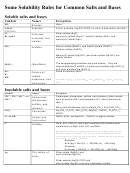
Solubility Guidelines For Common Ionic Compounds In Water
ADVERTISEMENT
11.1
Solubility Guidelines for Common Ionic Compounds in
Water
Soluble Compounds
Important Exceptions
Compounds containing:
-
NO
None
3
-
C
H
O
None
2
3
2
-
-
-
+
2
2+
Cl
, Br
, I
Ag
, Hg
+, Pb
2
2-
2+
2+
2+
2+
2+
SO
Ca
, Sr
, Ba
, Hg
, Pb
4
2
Insoluble Compounds
Important Exceptions
Compounds containing:
2-
+
S
NH
, alkali metal cations,
4
2+
2+
2+
Ca
, Sr
, Ba
2-
+
CO
NH
, alkali metal cations
3
4
3-
+
PO
NH
, alkali metal cations
4
4
2+
2+
OH-
alkali metal cations, Ca
, Sr
,
2+
Ba
+
+
+
+
+
Alkali metal cations = Li
, Na
, K
, Cs
, Rb
For this table, insoluble means a solubility of less than
0.01 M.
CHEM 101 Lecture 11
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3