Structure Of Proteins And Chemical Bonding
ADVERTISEMENT
January 2001
Number 80
Structure and Biological Functions of Proteins
By studying this Factsheet the student should gain knowledge and understanding of:
• The primary, secondary, tertiary and quarternary structure of proteins, including fibrous and globular types.
• The effect of pH on amino acids and proteins.
• Denaturation by extremes of pH or temperature.
• The biological functions of proteins, enzymes, hormones, carriers, membrane proteins, including structural, contraction, protection
(antibodies), osmotic and buffering roles.
For a full description of the chemical bonds referred to in this Factsheet the
Remember - the sequence of amino acids in the polypeptide is governed
student should refer to Factsheet No.78, September 2000, Chemical
by the sequence of codons in the gene that assembles that polypeptide by
Bonding in Biological Molecules.
using the messenger RNA/transfer RNA/ribosome mechanism.
Remember - amino acids are made in autotrophic green plants as
products of photosynthesis, and then are assembled into proteins.
The polypeptide chain is folded to make particular three dimensional shapes
Heterotrophic organisms gain their amino acids and proteins from
known as the 'secondary structure of the protein'. These shapes may either
plants through food chains in the case of animals, or in decay processes
be of the alpha-helix type or the beta-pleated-sheet type. They are
in the case of bacteria and fungi.
characteristic of fibrous type structural proteins. The secondary structure
may be further folded tightly to give the 'tertiary structure of the protein'.
This is characteristic of globular type proteins such as enzymes and
The structure of proteins
antibodies. Secondary and tertiary structures are still single polypeptides.
Twenty types of amino acid occur which form the 'building blocks' of
The 'quaternary structure of a protein' is the way in which polypeptides
proteins. Amino acids join together by peptide bonds, formed by
(in secondary or tertiary form) join together to form proteins.
condensation between the acid group of one amino acid and the amine
group of the other amino acid (Fig 1). When two amino acids join in this
The secondary, tertiary and quaternary structures are not loosely, randomly
way the product is a dipeptide. Many amino acids joined in this way make
folded structures but are precisely shaped and cross-bonded by ionic,
up a polypeptide.
hydrogen, sulphur and peptide bonds. These are formed between reactive
groups in the amino acid side chains. (The core acid and amine groups of the
Remember - condensation is the joining of molecules by the removal of
amino acids are already involved in joining the amino acids by peptide
water and is used in many synthetic processes. The reverse process is
links). Figs 2 and 3 show three dimensional forms of secondary, tertiary
hydrolysis which is the splitting of molecules by the addition of water
and quaternary polypeptides and protein molecules.
and is used in digestion.
Fig 2. Three dimensional forms of polypeptide- Secondary structures
Fig 1. Formation of a peptide bond between two amino acids
secondary structure - an alpha-helix
chain of amino acids
joined by peptide bonds
H
R
O
H
R
O
amino
C
C
C
N
N
C
cross bonds
acids
maintaining specific
H
H
OH
H
OH
H
shape of structure
condensation/synthesis
hydrolysis/digestion
H
O
secondary structure - a beta-pleated sheet
2
peptide bond
+
R
O
H
R
R
O
R
R
R
N
C
N
C
C
C
R
R
R
R
amino acid
H
OH
R
H
H
R
H
side chain
R
R = amino acid side chain
R
R
R
R
R
R
R
More amino acids can join by peptide bonds onto the ends of the dipeptide
R
resulting in the formation of a polypeptide. The polypeptide with its
three adjacent amino acid chains cross bonded and folded and
specific sequence of amino acids is called the 'primary structure of the
folded to form a beta-pleated sheet
protein'.
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1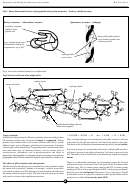 2
2 3
3 4
4








