Graphing Periodic Trends
ADVERTISEMENT
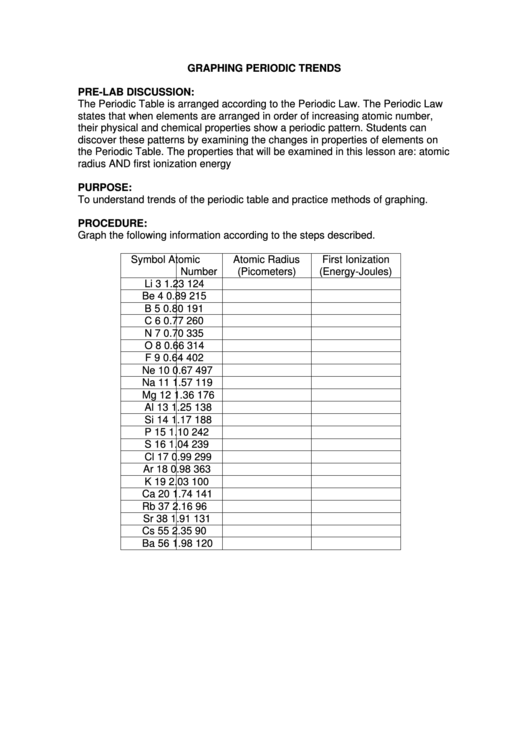
GRAPHING PERIODIC TRENDS
PRE-LAB DISCUSSION:
The Periodic Table is arranged according to the Periodic Law. The Periodic Law
states that when elements are arranged in order of increasing atomic number,
their physical and chemical properties show a periodic pattern. Students can
discover these patterns by examining the changes in properties of elements on
the Periodic Table. The properties that will be examined in this lesson are: atomic
radius AND first ionization energy
PURPOSE:
To understand trends of the periodic table and practice methods of graphing.
PROCEDURE:
Graph the following information according to the steps described.
Symbol
Atomic
Atomic Radius
First Ionization
Number
(Picometers)
(Energy-Joules)
Li
3
1.23
124
Be
4
0.89
215
B
5
0.80
191
C
6
0.77
260
N
7
0.70
335
O
8
0.66
314
F
9
0.64
402
Ne
10
0.67
497
Na
11
1.57
119
Mg
12
1.36
176
Al
13
1.25
138
Si
14
1.17
188
P
15
1.10
242
S
16
1.04
239
Cl
17
0.99
299
Ar
18
0.98
363
K
19
2.03
100
Ca
20
1.74
141
Rb
37
2.16
96
Sr
38
1.91
131
Cs
55
2.35
90
Ba
56
1.98
120
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








