Valence Electrons And Reactivity Student Journal Template
ADVERTISEMENT
Protons and Electrons
Matter and Energy
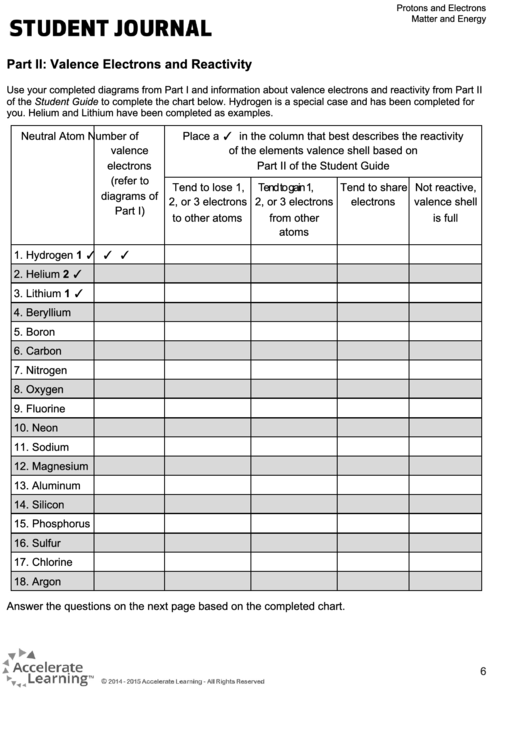
Part II: Valence Electrons and Reactivity
Use your completed diagrams from Part I and information about valence electrons and reactivity from Part II
of the Student Guide to complete the chart below. Hydrogen is a special case and has been completed for
you. Helium and Lithium have been completed as examples.
Neutral Atom
Number of
Place a ✓ in the column that best describes the reactivity
valence
of the elements valence shell based on
electrons
Part II of the Student Guide
(refer to
Tend to lose 1,
Tend to gain 1,
Tend to share
Not reactive,
diagrams of
2, or 3 electrons
2, or 3 electrons
electrons
valence shell
Part I)
to other atoms
from other
is full
atoms
1. Hydrogen
1
✓
✓
✓
2. Helium
2
✓
3. Lithium
1
✓
4. Beryllium
5. Boron
6. Carbon
7. Nitrogen
8. Oxygen
9. Fluorine
10. Neon
11. Sodium
12. Magnesium
13. Aluminum
14. Silicon
15. Phosphorus
16. Sulfur
17. Chlorine
18. Argon
Answer the questions on the next page based on the completed chart.
6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








