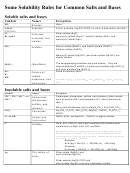
Some Review Problems For Exam 1 Chem 1a
ADVERTISEMENT
Some Review Problems for Exam 1 – Chem 1A
(Note: this set of problems is not comprehensive. Make sure to review everything on the
“Things to Know” handout and the lecture notes.)
1.
For the reaction:
AlCl
+
Mg
Al
+
MgCl
→
3 (aq)
(s)
(s)
2 (aq)
a. How many moles of magnesium are required to react with 5.0 moles of
aluminum chloride?
b. If you start with 50.0 mg of magnesium, how many moles of aluminum can be
formed?
c. Starting with 3.00 g AlCl
, calculate the mass of Mg needed to react.
3
24
d. If you would like to produce 5.0 × 10
atoms of Al, how many cubic
3
centimeters of Mg must you start with, if Mg has a density of 1.738 g/cm
?
2.
Calculate the mass percent of each element in Fe
(C
O
)
.
2
2
4
3
3.
Write the balanced equation for the combination reaction that would occur
between aluminum metal and fluorine gas.
4.
a. If you mix 20.0 grams S
and 10.0 grams Cl
, what mass of S
Cl
will be
8
2
2
2
formed?
S
+ Cl
→ S
Cl
8 (s)
2 (g)
2
2 (g)
b. What mass of Cl
is needed to produce 85.0 kg of disulfur dichloride?
2
c. If the percent yield of this reaction is known to be 86.5 %, what mass of S
8
should you start with to obtain 25.0 g of disulfur dichloride?
85
87
5.
Rubidium (Rb) consists of two isotopes,
Rb and
Rb. Which is more abundant?
Explain how you can tell.
6.
How many hydrogen atoms are in 2.0 nanograms (ng) of pentane (C
H
)?
5
12
7.
A compound containing C, H, and O is analyzed by combusting the compound
and collecting the water and carbon dioxide produced. If 2.317 g of the compound
produces 6.111 g CO
and 1.390 g H
O, determine the empirical formula of this
2
2
compound.
8.
If 5.00 g of Al(OH)
and 8.00 g of HCl are mixed,
3
a. Write the balanced equation for this reaction.
b. What mass of water will be obtained?
c. What is the mass of the excess reactant remaining?
9.
If 50.0 grams of octane (C
H
) was burned in excess oxygen, what masses of
8
18
carbon dioxide and water would be produced?
10.
The density of CO
at room temperature and pressure is 1.80 g/L. How many
2 (g)
CO
molecules are there per cubic inch of volume? (1 in = 2.54 cm)
2
11.
Silicon has three stable isotopes. Their masses and percent natural abundances are
shown below. Calculate the weighted-average atomic mass of silicon.
28
Si
27.97693 amu
92.23 %
29
Si
28.97649 amu
4.67 %
30
Si
29.97376 amu
3.10 %
12.
Write the balanced net ionic equations for the following reactions. Also be able to
write the molecular and total ionic equations.
CuSO
+ Na
PO
→
4 (aq)
3
4 (aq)
Ba(OH)
+ HC
H
O
→
2 (aq)
2
3
2 (aq)
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3