Chemistry Worksheet - Samples Of Every Kind Of Problem
ADVERTISEMENT
Chemistry Unit 8 Worksheet 4
Samples of Every Kind of Problem
On a separate sheet of paper, write a complete solution to each of the problems below.
Follow the procedure outlined in class. Be sure to circle your final answer.
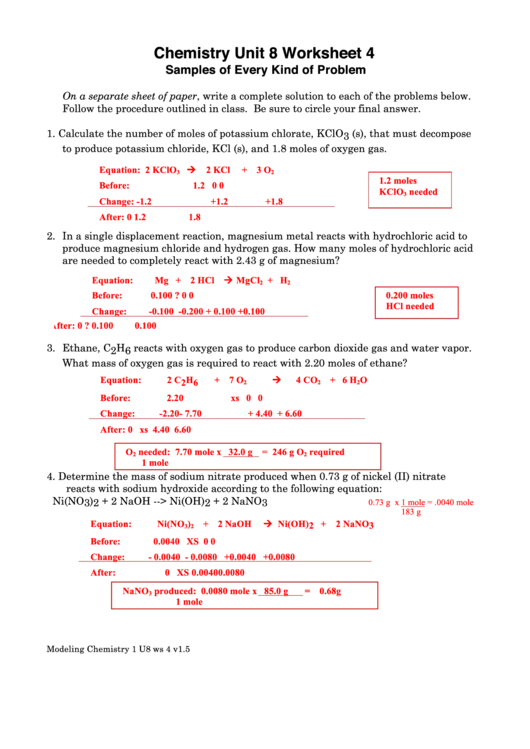
1. Calculate the number of moles of potassium chlorate, KClO 3 (s), that must decompose
to produce potassium chloride, KCl (s), and 1.8 moles of oxygen gas.
Equation:
2 KClO
2 KCl
+ 3 O
3
2
1.2 moles
Before:
1.2
0
0
KClO
needed
3
Change:
-1.2
+1.2
+1.8
After:
0
1.2
1.8
2. In a single displacement reaction, magnesium metal reacts with hydrochloric acid to
produce magnesium chloride and hydrogen gas. How many moles of hydrochloric acid
are needed to completely react with 2.43 g of magnesium?
Mg + 2 HCl MgCl
Equation:
+ H
2
2
Before:
0.100
?
0
0
0.200 moles
HCl needed
Change:
-0.100
-0.200 +
0.100 +
0.100
After:
0
?
0.100
0.100
3. Ethane, C 2 H 6 reacts with oxygen gas to produce carbon dioxide gas and water vapor.
What mass of oxygen gas is required to react with 2.20 moles of ethane?
Equation:
2 C 2 H 6
+ 7 O
4 CO
+ 6 H
O
2
2
2
Before:
2.20
xs
0
0
Change:
-2.20
- 7.70
+ 4.40
+ 6.60
After:
0
xs
4.40
6.60
O
needed: 7.70 mole x 32.0 g = 246 g O
required
2
2
1 mole
4. Determine the mass of sodium nitrate produced when 0.73 g of nickel (II) nitrate
reacts with sodium hydroxide according to the following equation:
Ni(NO 3 ) 2 + 2 NaOH --> Ni(OH) 2 + 2 NaNO 3
0.73 g x 1 mole = .0040 mole
183 g
Equation:
Ni(NO
)
+ 2 NaOH
Ni(OH) 2 + 2 NaNO 3
3
2
Before:
0.0040
XS
0
0
Change:
- 0.0040
- 0.0080
+0.0040
+0.0080
After:
0
XS
0.0040
0.0080
NaNO
produced: 0.0080 mole x 85.0 g
= 0.68 g
3
1 mole
Modeling Chemistry
1
U8 ws 4 v1.5
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








