Real Gases Worksheet
ADVERTISEMENT
C h e m g u i d e – a n s w e r s
REAL GASES
1. a) The graphs would be the same horizontal straight line with a compression factor of 1 at all
temperatures. For an ideal gas, pV = nRT. So pV/nRT will always be exactly 1.
b) An ordinary lab pressure would be around 1 bar, and the temperature would probably be a bit less
than 300 K. Under those conditions, the graphs show that the compression factor is close to 1, and
so pV = nRT is a reasonable approximation. If a gas obeys the ideal gas equation, then we must
count it as ideal under those conditions.
c) In working out the ideal gas equation, Kinetic Theory assumes that the whole volume of the
container is available to the molecules to move around in. In a real gas, some of the space is taken
up by the molecules themselves. That means that the measured volume of the container that you
put into the expression pV/nRT is bigger than the actual space available. As you compress the gas,
the molecules take up an increasing percentage of the available space, and so the error gets greater
as pressure increases.
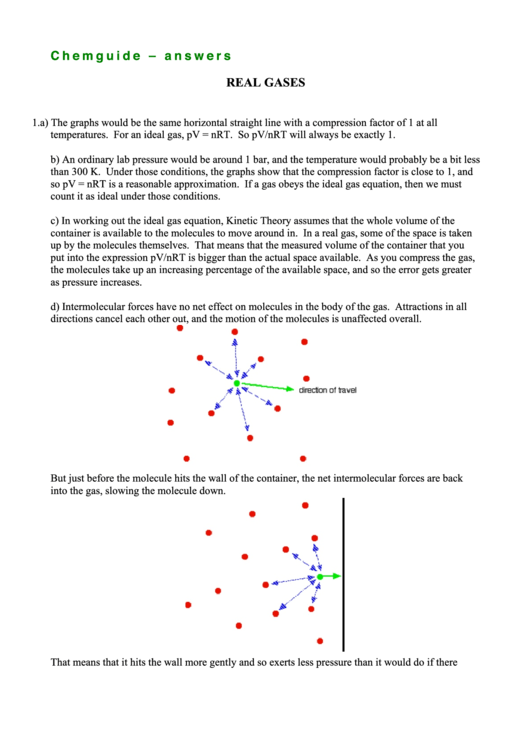
d) Intermolecular forces have no net effect on molecules in the body of the gas. Attractions in all
directions cancel each other out, and the motion of the molecules is unaffected overall.
But just before the molecule hits the wall of the container, the net intermolecular forces are back
into the gas, slowing the molecule down.
That means that it hits the wall more gently and so exerts less pressure than it would do if there
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








