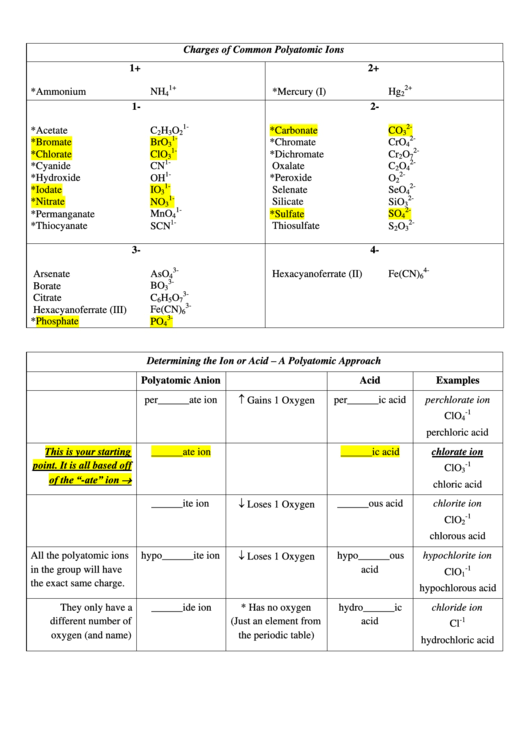
Charges Of Common Polyatomic Ions Chart
ADVERTISEMENT
Charges of Common Polyatomic Ions
1+
2+
1+
2+
*Ammonium
NH
*Mercury (I)
Hg
4
2
1-
2-
1-
2-
*Acetate
C
H
O
*Carbonate
CO
2
3
2
3
1-
2-
*Bromate
BrO
*Chromate
CrO
3
4
1-
2-
*Chlorate
ClO
*Dichromate
Cr
O
3
2
7
1-
2-
*Cyanide
CN
Oxalate
C
O
2
4
1-
2-
*Hydroxide
OH
*Peroxide
O
2
1-
2-
*Iodate
IO
Selenate
SeO
3
4
1-
2-
*Nitrate
NO
Silicate
SiO
3
3
1-
2-
*Permanganate
MnO
*Sulfate
SO
4
4
1-
2-
*Thiocyanate
SCN
Thiosulfate
S
O
2
3
3-
4-
3-
4-
Arsenate
AsO
Hexacyanoferrate (II)
Fe(CN)
4
6
3-
Borate
BO
3
3-
Citrate
C
H
O
6
5
7
3-
Hexacyanoferrate (III)
Fe(CN)
6
3-
*Phosphate
PO
4
Determining the Ion or Acid – A Polyatomic Approach
Polyatomic Anion
Acid
Examples
Gains 1 Oxygen
per______ate ion
per______ic acid
perchlorate ion
-1
ClO
4
perchloric acid
______ate ion
______ic acid
This is your starting
chlorate ion
-1
point. It is all based off
ClO
3
of the “-ate” ion
chloric acid
Loses 1 Oxygen
______ite ion
______ous acid
chlorite ion
-1
ClO
2
chlorous acid
Loses 1 Oxygen
All the polyatomic ions
hypo______ite ion
hypo______ous
hypochlorite ion
in the group will have
acid
-1
ClO
1
the exact same charge.
hypochlorous acid
They only have a
______ide ion
* Has no oxygen
hydro______ic
chloride ion
different number of
(Just an element from
acid
-1
Cl
oxygen (and name)
the periodic table)
hydrochloric acid
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








