Dialysis Event Form
ADVERTISEMENT
Form Approved
Dialysis Event
OMB No. 0920-0666
Exp. Date: 12/31/2018
Complete this form as indicated by the Dialysis Event Protocol
Instructions for this form are available at
Page 1 of 4
*required for saving
Facility ID:
Event ID #:
*Patient ID:
Social Security #:
Secondary ID #:
Medicare #:
Patient Name, Last:
First:
Middle:
*Gender: F
M
Other
*Date of Birth:
Ethnicity (Specify):
Race (Specify):
*Event Type: DE – Dialysis Event
*Date of Event:
*Location:
Yes
No
*Was the patient admitted/readmitted to the dialysis facility on this dialysis event date?
Yes
No
*Transient Patient
Risk Factors
*Vascular accesses: (check all that apply)
*Access placement date (mm/yyyy):
Fistula
Unknown
_____ /_________
Yes
No
Buttonhole?
Graft
Unknown
_____ /_________
Tunneled central line
Unknown
_____ /_________
Nontunneled central line
Unknown
_____ /_________
Other vascular access device, specify:
Unknown
_____ /_________
Yes No
Is this a catheter-graft hybrid?
Vascular access comment: __________________________________________________________
Patient’s dialyzer is reused?
Yes
No
Event Details
*Specify Dialysis Event: (check at least one)
IV antimicrobial start
Yes No
*Was vancomycin the antimicrobial used for this start?
Was this a new outpatient start or a continuation of an inpatient course?
New antimicrobial start
Continuation of antimicrobial
Positive blood culture (*specify organism and antimicrobial susceptibilities on pages 2-3)
*Suspected source of positive blood culture (check one):
Vascular access
A source other than the vascular access
Contamination
Uncertain
*Where was this positive blood culture collected?
Dialysis clinic
Hospital
Other location
or E.D.
(on the day of or the day following admission)
Pus, redness, or increased swelling at vascular access site
*Check the access site(s) with pus, redness, or increased swelling:
Fistula
Graft
Tunneled
Nontunneled
Other vascular
central
line
central line
access device
*Specify Problem(s): (check one or more)
Fever ≥37.8°C (100°F) oral
Chills or rigors
Drop in blood pressure
Wound (NOT related to vascular access) with pus or increased redness
Urinary tract infection
Cellulitis (skin redness, heat, or pain without open wound)
Pneumonia or respiratory infection
Other problem (specify): __________________________________________________________
None
Yes
No
Unknown
*Specify Outcomes:
Loss of vascular access
Yes
No
Unknown
Hospitalization
Yes
No
Unknown
Death
Assurance of Confidentiality: The voluntarily provided information obtained in this surveillance system that would permit identification of any individual or institution is
collected with a guarantee that it will be held in strict confidence, will be used only for the purposes stated, and will not otherwise be disclosed or released without the consent
of the individual, or the institution in accordance with Sections 304, 306 and 308(d) of the Public Health Service Act (42 USC 242b, 242k, and 242m(d)).
Public reporting burden of this collection of information is estimated to average 25 minutes per response, including the time for reviewing instructions, searching existing data
sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not
required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect
of this collection of information, including suggestions for reducing this burden to CDC, Reports Clearance Officer, 1600 Clifton Rd., MS D-74, Atlanta, GA 30333, ATTN:
PRA (0920-0666).
CDC 57.502 (Front) Rev 9, v8.5
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Medical
 1
1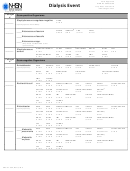 2
2 3
3 4
4








