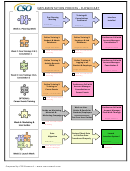
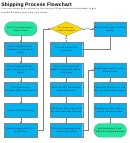
Research Process Flowchart Page 14
ADVERTISEMENT
7. Collect and collate the data
Issues to consider
Beware of biases: yours and/or other researchers’
Seek statistical advice if necessary
Conduct Issues
Researchers bear the day-to-day responsibility for the conduct of
research in terms of:
Ensuring that research follows the agreed protocol (or
proposal).
Making sure that participants receive appropriate care while
involved in research.
Protecting the integrity and confidentiality of clinical and other
records and data generated by the research.
Reporting any failures in these respects, any adverse drug
reactions and other events or suspected misconduct through
the appropriate systems.
Data collected in the course of research must be retained for an
appropriate period to allow further analysis by the original or other
research teams subject to consent, and to support monitoring of
good research practice by regulatory and other authorities.
Data Protection
Data Protection Act stipulates that the appropriate use and
protection of patient data is paramount in the research setting.
All those involved in research must be aware of their legal and
ethical duties, particularly in terms of ensuring confidentiality of
personal information about living or deceased participants.
When collecting and storing data on human participants, the
following should be considered:
Identities should be disguised by use of codes (do not use
initials!)
Any details should be anonymised
Use of patient-identifiable information should be avoided
unless absolutely necessary
If unavoidable, only minimum necessary patient-identifiable
information should be used
Access to patient-identifiable information should be on a strict
need to know basis.
More information can be found on
Data Protection in Healthcare
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1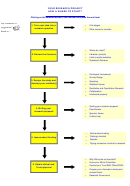 2
2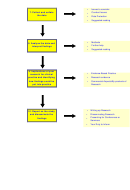 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22