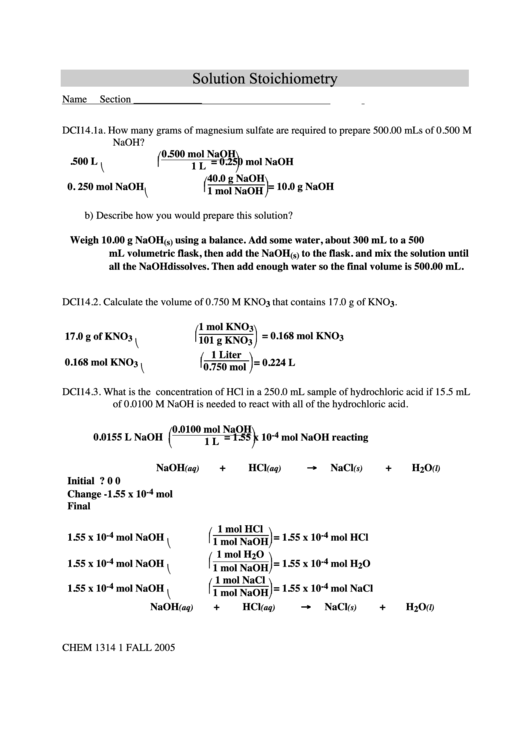
Solution Stoichiometry Worksheet
ADVERTISEMENT
Solution Stoichiometry
Name
Section _____________
DCI14.1a. How many grams of magnesium sulfate are required to prepare 500.00 mLs of 0.500 M
NaOH?
0.500 mol NaOH
⎛
⎞
.500 L
⎜
⎟
= 0.250 mol NaOH
1 L
⎝
⎠
40.0 g NaOH
⎛
⎞
0. 250 mol NaOH
1 mol NaOH = 10.0 g NaOH
⎜
⎟
⎝
⎠
b) Describe how you would prepare this solution?
Weigh 10.00 g NaOH
using a balance. Add some water, about 300 mL to a 500
(s)
mL volumetric flask, then add the NaOH
to the flask. and mix the solution until
(s)
all the NaOH dissolves. Then add enough water so the final volume is 500.00 mL.
DCI14.2.
Calculate the volume of 0.750 M KNO
that contains 17.0 g of KNO
.
3
3
1 mol KNO
⎛
⎞
3
17.0 g of KNO
⎜
⎟
= 0.168 mol KNO
3
3
101 g KNO
⎝
⎠
3
1 Liter
⎛
⎞
0.168 mol KNO
= 0.224 L
⎜
⎟
3
0.750 mol
⎝
⎠
DCI14.3.
What is the concentration of HCl in a 250.0 mL sample of hydrochloric acid if 15.5 mL
of 0.0100 M NaOH is needed to react with all of the hydrochloric acid.
0.0100 mol NaOH
⎛
⎞
-4
0.0155 L NaOH
⎜
⎟
= 1.55 x 10
mol NaOH reacting
1 L
⎝
⎠
NaOH
+
HCl
NaCl
+
H
O
→
(aq)
(aq)
(s)
(l)
2
Initial
?
0
0
-4
Change
-1.55 x 10
mol
Final
1 mol HCl
⎛
⎞
-4
-4
1.55 x 10
mol NaOH
⎜
1 mol NaOH = 1.55 x 10
⎟
mol HCl
⎝
⎠
1 mol H
O
⎛
⎞
2
-4
-4
mol NaOH ⎝ ⎜
⎟
1.55 x 10
1 mol NaOH = 1.55 x 10
mol H
O
2
⎠
1 mol NaCl
⎛
⎞
-4
-4
1.55 x 10
mol NaOH
1 mol NaOH = 1.55 x 10
mol NaCl
⎜
⎟
⎝
⎠
NaOH
+
HCl
NaCl
+
H
O
→
(aq)
(aq)
(s)
(l)
2
CHEM 1314
1
FALL 2005
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4