Stoichiometry Notes
ADVERTISEMENT
Notes
Chapter 9 – Stoichiometry
Notes
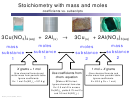
Interpret the following equation:
N
+
3H
2NH
in terms of:
→
2 (g)
2 (g)
3 (g)
makes
Numbers of moles
1 mol N
+
3 mol H
2 moles NH
→
→
2
2
3
yields
Numbers of molecules→
23
23
23
6.02 X 10
molecules N
+ 3(6.02 X 10
) molecules H
2(6.02 X 10
) molecules NH
→
2
2
3
Volumes of gases
22.4 L of N
+
3(22.4 L) of H
2(22.4L) NH
→
→
2
2
3
Or
22.4 L of N
+
67.2 L of H
44.8 L of NH
→
2
2
3
Masses of gases
28 g of N
+
3 ( 2g) of H
2 ( 17 g) of NH
→
→
2
2
3
Or
28 g of N
+
6 g of H
34 g of NH
→
2
2
3
We said above that 1 mole of N
will produce 2 moles of NH
. But what if I give you 0.6 moles
2
3
of N
? How many moles of NH
will you make?
2
3
N
+
3H
2NH
Work from moles of N
to
→
2 (g)
2 (g)
3 (g)
2
0.6 mol
? mol
moles of NH
.
3
Use dimensional analysis and
begin with the number you were
0.6 mol N
2 mol NH
=
1.2 mol NH
2
3
3
given in the problem.
1 mol N
2
In the equation below, how many moles of Aluminum are needed to form 3.7 moles of Al
O
?
2
3
4Al
+
3O
2Al
O
Work from moles of Al
O
to
→
2
2
3
2
3
? mol
3.7 mol
moles of Al.
Use dimensional analysis and
begin with the number you were
3.7 mol Al
O
4 mol Al
= 7.4 mol Al
2
3
given in the problem.
2 mol Al
O
2
3
In the above equation, how many moles of Oxygen are required to react completely with 14.8
moles of Aluminum?
4Al
+
3O
2Al
O
Work from moles of Al to
→
2
2
3
14.8 mol
? mol
moles of O
.
2
Use dimensional analysis and
begin with the number you were
14.8 mol Al
3 mol O
= 11.1 mol O
2
2
given in the problem.
4 mol Al
How many moles of Al
O
are formed when 0.78 mol O
reacts with Aluminum?
2
3
2
4Al
+
3O
2Al
O
Work from moles of O
to
→
2
2
3
2
0.78 mol
? mol
moles of Al
O
.
2
3
Use dimensional analysis and
begin with the number you were
0.78 mol O
2 mol Al
O
= 0.52 mol Al
O
2
2
3
2
3
given in the problem.
3 mol O
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3