Chemistry Worksheet Page 2
ADVERTISEMENT
a) Na
O =sodium oxide
b) P
O
= tetraphosphorus decoxide
2
4
10
c) MgCl
= magnesium dichloride
d) CO
= carbon dioxide
2
2
For ionic compounds, we don’t use the prefixes like “di”. So C) is wrong.
C
(10) Consider the following electronegativities: H = 2.2, Li =1.0, B =2.0, C= 2.5, O =3.5.
Choose the correct statement below:
a) The C-H bond is polar b) The Li-B bond is nonpolar c) The O-H bond is polar d) The C-O bond is ionic.
Only C) has an ∆eneg which matches. ∆eneg for O-H bond is 3.5-2.2 = 1.3 which is between .5 and 1.9. That makes it a polar bond.
3-
C
(11) The correct name for the polyatomic anion, PO
is:
4
a) phosphorus tetroxide b) potassium tetroxide
c) phosphate
d) phosphite
Only C is correct. Consult the table for names of polyatomic ions.
A
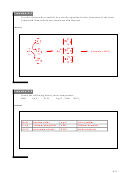
(12) Consider the molecule carbon tetrafluoride. Choose the correct molecular structure:
a) tetrahedral
b) linear
c) bent
d) trigonal planar
Since there are 4 atoms around the carbon, the arrangement is tetrahedral.
Partial Periodic Table
1.0
4.0
H
He
1
2
6.9
9.0
10.8
12.0
14.0
16.0
19.0
20.2
Li
Be
B
C
N
O
F
Ne
3
4
5
6
7
8
9
10
23.0
24.3
27.0
28.1
31.0
32.1
35.5
39.9
Na
Mg
Al
Si
P
S
Cl
Ar
11
12
13
14
15
16
17
18
39.1
40.1
45.0
47.9
50.9
52.0
54.9
55.8
58.9
58.7
63.5
65.4
69.7
72.6
74.9
79.0
79.9
83.8
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
85.5
87.6
Rb
Sr
37
38
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2