Anion Analysis
ADVERTISEMENT
Chemistry 112: Anion Analysis
Page 7
ANION ANALYSIS
M
uch of the work you will be doing in the Chemistry 112 laboratory will
be concerned with identifying positive and negative ions, that is, cations
and anions, in solutions whose composition is unknown. This procedure is
called QUALITATIVE ANALYSIS.
The modern chemist frequently wishes to identify the constituents in a very
small amount of substance, and so he depends heavily on instrumental meth-
ods of analysis. While the procedures you will use do not use fancy and expen-
sive instruments, your methods are still very effective in determining the
major components of systems containing common inorganic ions. Because
anion analysis is somewhat simpler than cation analysis, we shall begin our
work in qualitative analysis with methods of identifying four common anions
in solution:
3-
-
PO
, phosphate
Cl
, chloride
4
2-
-
SO
, sulfate
NO
, nitrate
4
3
After having determined the chemical reactions of the individual ions,
you will be asked to identify the ions present in an unknown mixture.
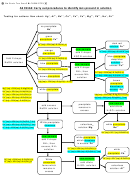
CHEMICAL REACTIONS OF INDIVIDUAL ANIONS
1. The BaCl
Test
2
Take a set of four small test tubes. After cleaning them, label them 1 through 4,
Be sure to record the
and place 4-5 drops of one of the known solutions in each tube as follows:
results of your tests in
Test tube
Known Solution
your notebook.
3-
1
PO
, phosphate
4
2-
2
SO
, sulfate
4
-
3
Cl
, chloride
-
4
NO
, nitrate
3
Next, make each solution slightly basic by adding 5 M ammonia (NH
)
3
dropwise. Making sure the solution is thoroughly mixed, test the basicity of
The ammonia bottle may
the solution with litmus paper as demonstrated by your instructor.
be labeled either with the
formula NH
or, less cor-
When the solutions are basic, note any changes that have occurred, and
3
rectly, as NH
OH.
4
enter your observations in your lab book. Next, add 2-3 drops of 0.2 M BaCl
2
2+
to form precipitates between Ba
and some of the anions.
To test for a basic solu-
(aqueous) + anion(aqueous) → [Ba(anion)](solid)
2+
Ba
tion, use red litmus
Record observations on the color and texture or appearance of the pre-
paper. It will turn blue if
the solution basic. Just
cipitates in your notebook. It is best to draw a table in your notebook some-
remember: blue = base.
thing like that below.
Some of the precipitates you have formed will dissolve in acid. In each
case where a precipitate has formed with BaCl
, make the solution acidic with
2
6 M HCl (blue litmus paper should turn red in acid). Be sure to mix the solu-
tion well after adding acid! (The most common error made in qualitative analysis
laboratory is to fail to mix solutions completely!) Record your observations. (If
you had made a table as described above, you can add your observations on
Revised: December 2005
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4