Writing Formulae For Covalent Compounds
ADVERTISEMENT
W
riting formulae for covalent compounds
1
Covalent compounds are (usually) made up of atoms of non-metal elements.
The formula for covalent compounds can be found by drawing electron sharing diagrams.
These can be based on the sharing of electron pairs by the merging of outer electron shells.
The following is a quick way to write the formula for a covalent compound. It is based on the
number of bonds that an atom forms. This is equal to the number of extra electrons that an
atom requires to reach the same electron arrangement as a noble gas.
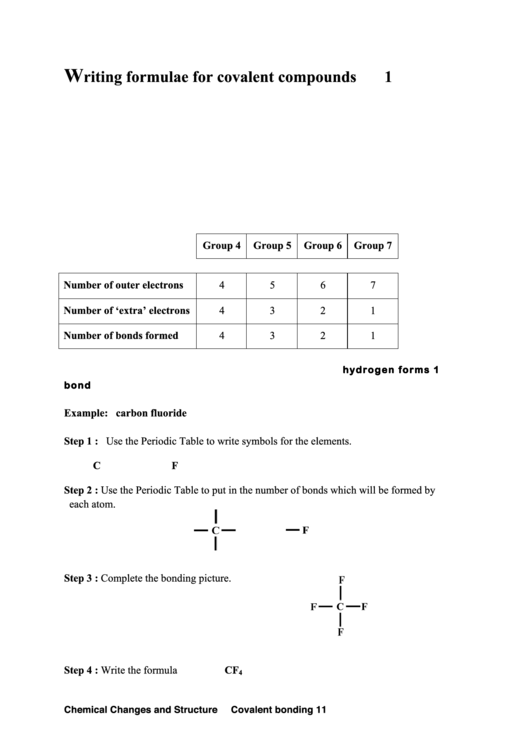
Group 4
Group 5
Group 6
Group 7
Number of outer electrons
4
5
6
7
Number of ‘extra’ electrons
4
3
2
1
Number of bonds formed
4
3
2
1
An atom of hydrogen is 1 electron short of an atom of helium so hydrogen form s 1
bond.
Example: carbon fluoride
Step 1 :
Use the Periodic Table to write symbols for the elements.
C
F
Step 2 :
Use the Periodic Table to put in the number of bonds which will be formed by
each atom.
Step 3 :
Complete the bonding picture.
Step 4 :
Write the formula
CF
4
Chemical Changes and Structure
Covalent bonding 11
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2