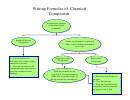
Writing Formulas Flow Chart Page 2
ADVERTISEMENT
Is the formula an element?
yes
no
Is it a diatomic element ( hydrogen
Does the name have prefixes of mono, di, tri, tetra
– H
, nitrogen – N
, oxygen – O
,
, etc.? If it does (except for dihydrogen phosphate,
2
2
2
fluorine – F
, chlorine – Cl
,
dihydrogen phosphite or dichromate) simply use
2
2
bromine – Br
, iodine – I
, astatine
the prefixes to write the formula. For help with
2
2
– At
)
prefixes look at your Naming Compounds Flow
2
Chart.
Example: dinitrogen trioxide is N
O
2
3
carbon dioxide is CO
2
If it is not a diatomic element the
sulfur hexafluoride is SF
6
formula is just the symbol.
Example: sodium is Na, silver is
Ag, tin is Sn. Notice that elements
ELSE
do not have a charge.
1. Write the formula for the cation and then the formula for the anion (see other side for help).
2. Use subscripts to make the charges add to zero (the positive charge of the cation must cancel
the negative charge of the anion. Do not write subscripts of one (the number one is “implied” just
like in algebra – x is really 1x, the one is implied). Because the total charge is zero there should
be no charges left in the formula.
3. If the ion needs subscripts and is a polyatomic ion then put parentheses around the ion before
the subscript.
1+
2-
Examples: lithium sulfate is Li
SO
because lithium ion is Li
and sulfate is SO
because two
2
4
4
lithium ions at a 1- charge are needed to “balance” the sulfate ion at a 2- charge.
1+
2-
ammonium carbonate is (NH
)CO
because ammonium ion is NH
and carbonate ion is CO
.
4
3
4
3
because two ammonium ions are needed to “balance” the carbonate ion and ammonium ion is a
poly atomic ion parentheses are used around the ammonium ion.
3+
2-
iron (III) bisulfite is Fe(HSO
)
Iron (III) ion is Fe
. Sulfate ion is SO
so bisulfate ion is
3
3
4
1-
HSO
. Combining them to get a zero charge requires 3 bisulfate ions for the one iron (III) ion.
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2