Amides Polyamides
ADVERTISEMENT
C h e m g u id e – q u e s t i o n s
AMIDES: POLYAMIDES
Before you do these questions, find out exactly what you need to know. There is no point in
answering questions about nylon-6 or Kevlar if all you need is nylon-6,6.
1. Nylon-6,6 is made from the monomers hexanedioic acid and 1,6-diaminohexane (also called
hexane-1,6-diamine).
a) Draw the structures of the two monomers.
b) Draw a short length of the polymer chain produced when the monomers react.
c) The formation of nylon-6,6 is an example of condensation polymerisation. Use the structures
you have drawn above to explain what this means.
d) Nylon-6,6 is often made in the lab using the “nylon rope trick”. This involves a solution of
hexanedioyl dichloride in an organic solvent, and a solution of 1,6-diaminohexane in water, one
floating on top of the other. The nylon-6,6 forms at the boundary of the two liquids, and the layer
can be pulled out into a long “rope” using tweezers. Chemically, what is the difference between this
reaction and the reaction involving hexanedioic acid and 1,6-diaminohexane?
e) Polyamides like nylon are attacked by dilute acids such as dilute sulphuric acid. Explain why
dilute acids make holes in nylon fabrics.
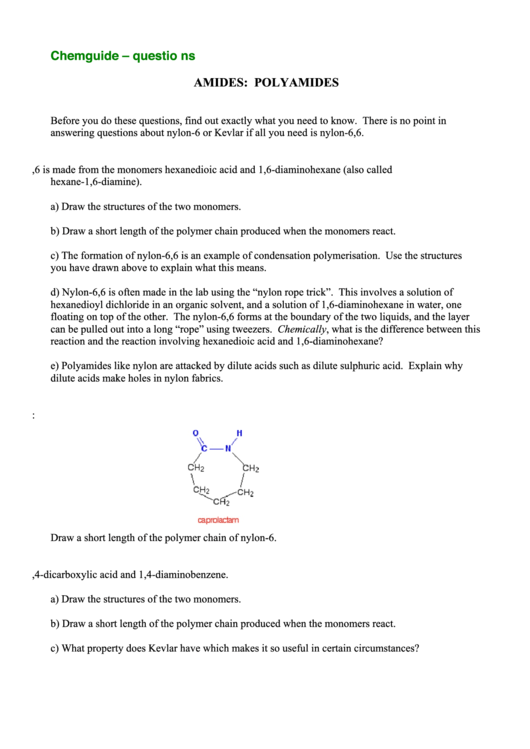
2. The single monomer that is used to make nylon-6 is caprolactam:
Draw a short length of the polymer chain of nylon-6.
3. Kevlar is made from the monomers benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene.
a) Draw the structures of the two monomers.
b) Draw a short length of the polymer chain produced when the monomers react.
c) What property does Kevlar have which makes it so useful in certain circumstances?
ADVERTISEMENT
0 votes
Related Articles
Related Categories
Parent category: Education
 1
1