Lewis Structures Worksheet
ADVERTISEMENT
Lewis Structures
Name:
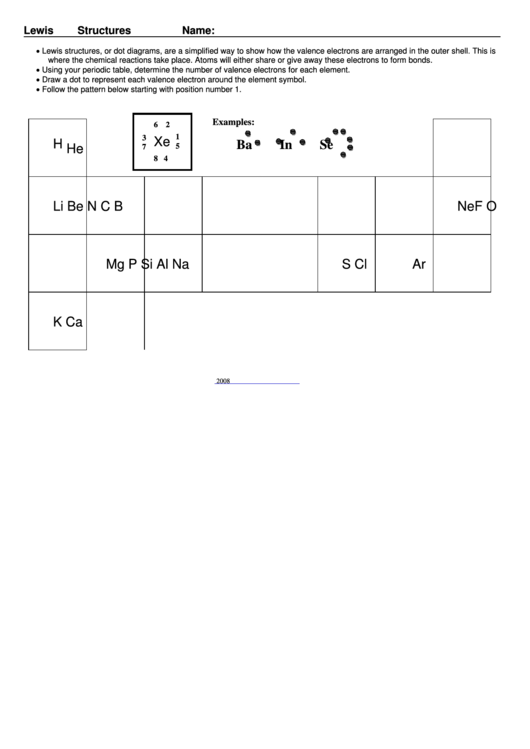
• Lewis structures, or dot diagrams, are a simplified way to show how the valence electrons are arranged in the outer shell. This is
where the chemical reactions take place. Atoms will either share or give away these electrons to form bonds.
• Using your periodic table, determine the number of valence electrons for each element.
• Draw a dot to represent each valence electron around the element symbol.
• Follow the pattern below starting with position number 1.
Examples:
6 2
1
3
Xe
H
Ba
In
Se
5
He
7
8 4
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
2008
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








