Chemistry Worksheets
ADVERTISEMENT
Chapter 4 Exercises
1.
Define the term “isoelectronic.”
14.
Determine the oxidation state of the underlined atom.
2.
Which of the following compounds are ionic?
a)
KMnO
b)
C
H
O
c)
CoPO
d)
Na
O
4
12
22
11
4
2
2
SiCl
ScCl
NCl
NH
Cl
a)
b)
c)
d)
4
3
3
4
15.
Determine the oxidation state of the underlined atom.
3.
Which of the following compounds are ionic?
a)
C
b)
LiAlH
c)
OF
d)
CaSiO
60
4
2
3
KCN
HNO
CoPO
NH
NO
a)
b)
c)
d)
2
4
4
2
16.
Name the following compounds:
4.
Metals and nonmetals tend to achieve noble gas configurations. In each
a)
CaCl
b)
Fe(NO
)
c)
K
CO
d)
CoCl
2
3
2
2
3
3
case, explain how they do it?
Name the following compounds:
17.
5.
What are the charges on the ions formed by the main group elements?
a)
Zn
(PO
)
b)
Ag
S
c)
Cr
O
d)
NH
Cl
3
4
2
2
2
3
4
Name the following ionic compounds using the “hydrogen” prefix for the
18.
6.
How many ions are in the formula of a compound composed of a 2A metal
anion:
and a 7A nonmetal? Give two examples of compounds with this type of
KHSO
NaH
PO
Li
HPO
Co(HSO
)
a)
b)
c)
d)
4
2
4
2
4
3
2
formula.
What two names can be used for Ca(HCO
)
?
19.
3
2
7.
Oxygen can have a positive oxidation state when bound to only one
element. What is the element? Use orbital energies to explain.
Predict the formulas of the arsenate and arsenite ions.
20.
Use orbital energies to explain why hydrogen is -1 when bound to metals
Predict the formulas of the vanadate and titanate ions.
8.
21.
and +1 when bound to nonmetals.
Which element in each pair would have the positive oxidation state.
9.
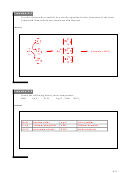
X
Use the energy diagram for the valence orbitals of atoms
X
,
a)
N & O
b)
Cl & P
c)
S & Sn
d)
K & N
Y
, and
Z
shown to the right in Exercises 22, 23, and 24.
Which element in each pair would have the positive oxidation state.
10.
a)
N & H
b) C & O
c)
S & Ca
d)
F & O
22.
Consider the compound formed between
X
and
Y.
Y
a)
What is the formula of the ionic compound formed
11.
Write electron configurations for the following ions.
2+
3+
3+
1-
between these two elements?
Ca
Ga
Co
I
a)
b)
c)
d)
b)
What is the oxidation state of
X
in the compound?
Write electron configurations for the following ions.
Z
12.
c)
What is the oxidation state of
Y
in the compound?
2-
3-
2+
1+
a)
Te
b)
P
c)
Pb
d)
In
23.
Consider the compound formed between
Y
and
Z
.
13.
Explain the following observations.
a)
What are the maximum and minimum oxidation states of
Y
?
1+
1+
K
is larger than Na
.
a)
b)
What are the maximum and minimum oxidation states of
Z
?
1+
1-
b)
Na is larger than Cl, but Na
is much smaller than Cl
.
c)
What is the formula of the compound that is most likely formed between
Lead forms two oxides, PbO and PbO
.
c)
2
atoms
Y
and
Z
in their maximum and minimum oxidation states?
24.
Elements X, Y, and Z are all main group elements. In which groups are
they located? (See Exercises 22 and 23.)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2