Covalent Compounds Worksheet Answer Key Template
ADVERTISEMENT
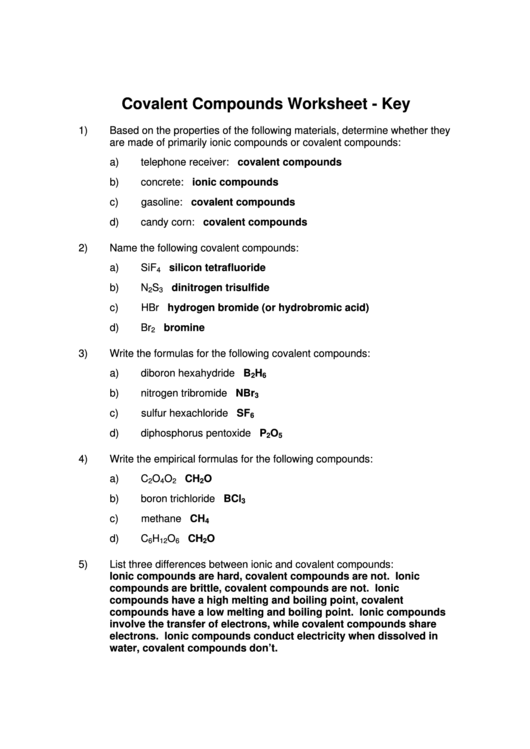
Covalent Compounds Worksheet - Key
1)
Based on the properties of the following materials, determine whether they
are made of primarily ionic compounds or covalent compounds:
a)
telephone receiver: covalent compounds
b)
concrete: ionic compounds
c)
gasoline: covalent compounds
d)
candy corn: covalent compounds
2)
Name the following covalent compounds:
a)
SiF
silicon tetrafluoride
4
b)
N
S
dinitrogen trisulfide
2
3
c)
HBr hydrogen bromide (or hydrobromic acid)
d)
Br
bromine
2
3)
Write the formulas for the following covalent compounds:
a)
diboron hexahydride B
H
2
6
b)
nitrogen tribromide NBr
3
c)
sulfur hexachloride SF
6
d)
diphosphorus pentoxide P
O
2
5
4)
Write the empirical formulas for the following compounds:
a)
C
O
O
CH
O
2
4
2
2
b)
boron trichloride BCl
3
c)
methane CH
4
d)
C
H
O
CH
O
6
12
6
2
5)
List three differences between ionic and covalent compounds:
Ionic compounds are hard, covalent compounds are not. Ionic
compounds are brittle, covalent compounds are not. Ionic
compounds have a high melting and boiling point, covalent
compounds have a low melting and boiling point. Ionic compounds
involve the transfer of electrons, while covalent compounds share
electrons. Ionic compounds conduct electricity when dissolved in
water, covalent compounds don’t.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








