Qualitative Analysis
ADVERTISEMENT
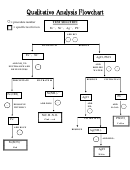
Qualitative Analysis
Chemical analysis can be either qualitative or quantitative in nature. In qualitative analysis we
want to know which elements or characteristic chemical species are present. In quantitative
analysis we are interested in the relative amounts of the components present. We will be
concerned with qualitative analysis in this laboratory. The classical qualitative analysis scheme
has been around for well over 100 years, but it continues to be an important part of any chemist's
training. It offers an effective means for presenting descriptive inorganic chemistry in the
laboratory and it illustrates not only descriptive chemistry, but also important chemical
principles, especially those involving ionic equilibria. I hope in the next few weeks that you will
gain new insight into these chemical principles.
Overview
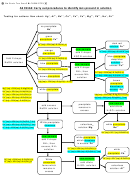
We will be developing an analytical scheme for the following ions:
+
2+
2+
2+
2+
2+
Ag
, Hg
, Pb
, Cu
, Hg
, Cd
2
Cations are separated into groups, using group reagents. The ions within a group are then
separated from each other. A confirmatory test is carried out for each ion when separations have
ensured that interfering ions have been removed. Usually this means isolation from all other
cations.
Separations are achieved by selective precipitation.
Subsequent separations may involve
differential solubility in water, acid, or base, or performing a redox reaction to convert an ion to
a different oxidation state. Confirmatory tests often involve a color change or formation of a
precipitate characteristic of a particular ion. Confirmatory tests are most often performed on an
ion separated from all others, and not on the original sample.
Laboratory Techniques for Semi-micro Qualitative Analysis
1.
Distilled water (DW) is used at all times, never tap water.
2.
It is essential that all glassware be clean, but not necessarily dry, before use.
3.
Care should be taken to label test tubes and beakers in order to avoid confusion.
4.
Do not place the reagent dropper tips into the mouth of the test tube. Hold them a little
above the mouth to avoid contamination.
5.
Estimate volumes (20 drops = 1 ml) whenever possible to save time.
6.
Always mix solutions thoroughly by flicking the test tube. Never shake with your finger
over the end.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2 3
3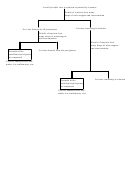 4
4