Chemical Bonding And Molecular Structure
ADVERTISEMENT
CHAPTER-4
CHEMICAL BONDING AND MOLECULAR STRUCTURE
OCTET RULE- During a chemical reaction the atoms tend to adjust their
-
electronic arrangement in such a way that they achieve 8 e
in their outermost
electron. This is called octet rule.
CHEMICAL BOND- the chemical force which keeps the atoms in any molecule
together is called a chemical bond.
IONIC BOND- The columbic force of attraction which holds the appositively
charged ions together is called an ionic bond. An ionic bond is formed by the
complete transfer of one or more electrons from the atom of a metal to an atom of
non- metal.
LATTICE ENTHALPY- The molar enthalpy change accompanying the complete
separation of the constituent particles that compose of the solids (such as ions for
ionic solid, molecules for molecular solids) under standard conditions is called
o
lattice enthalpy (∆
H
). The lattice enthalpy is a positive quantity.
l
ELECTRO VALENCY: The number of electrons lost or gain by an atom of an
element is called as electrovalency.
The element which give up electrons to form positive ions are said to have positive
valency, while the elements which accept electrons to form negative ions are said to
have negative valency.
FORMATION OF AN IONIC BOND: It is favoured by, (i) the low ionisation
enthalpy of a metallic element which forms the cations, (ii) High electron gain
enthalpy of non- metallic element which forms the anions, (iii) Large lattice
enthalpy i.e; the smaller size and the higher charge of the atoms.
COVALENCY:The number of electrons which an atom contributes towards
mutual sharing during the formation of a chemical bond called its covalency in that
compound.
SINGLE COVALENT BOND: A covalent bond formed by the mutual sharing of
one pair of electrons is called a single covalent bond, or simply a single bond. A
single covalent bond is represented by a small line (−) between the two atoms.
48
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3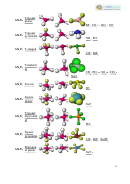 4
4








