Reading A Solubility Chart
ADVERTISEMENT
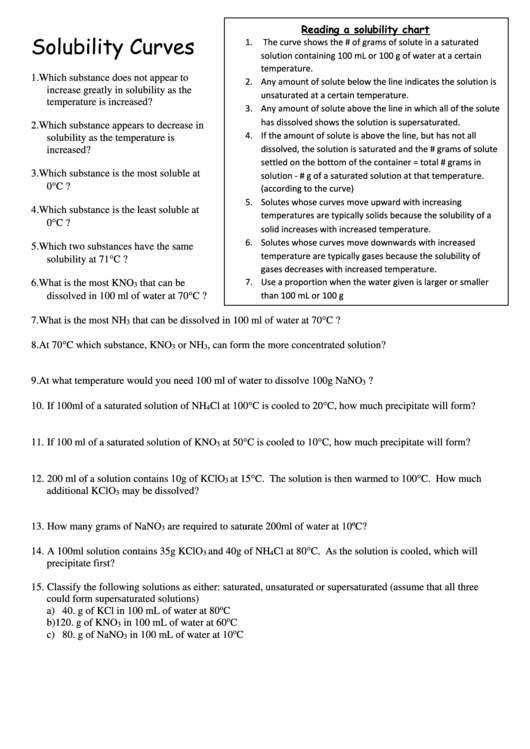
Reading a solubility chart
Solubility Curves
1.
The curve shows the # of grams of solute in a saturated
solution containing 100 mL or 100 g of water at a certain
temperature.
1. Which substance does not appear to
2. Any amount of solute below the line indicates the solution is
increase greatly in solubility as the
unsaturated at a certain temperature.
temperature is increased?
3. Any amount of solute above the line in which all of the solute
has dissolved shows the solution is supersaturated.
2. Which substance appears to decrease in
4. If the amount of solute is above the line, but has not all
solubility as the temperature is
dissolved, the solution is saturated and the # grams of solute
increased?
settled on the bottom of the container = total # grams in
3. Which substance is the most soluble at
solution - # g of a saturated solution at that temperature.
0C ?
(according to the curve)
5. Solutes whose curves move upward with increasing
4. Which substance is the least soluble at
temperatures are typically solids because the solubility of a
0C ?
solid increases with increased temperature.
6. Solutes whose curves move downwards with increased
5. Which two substances have the same
temperature are typically gases because the solubility of
solubility at 71C ?
gases decreases with increased temperature.
7. Use a proportion when the water given is larger or smaller
6. What is the most KNO
that can be
3
than 100 mL or 100 g
dissolved in 100 ml of water at 70C ?
7. What is the most NH
that can be dissolved in 100 ml of water at 70C ?
3
8. At 70C which substance, KNO
or NH
, can form the more concentrated solution?
3
3
9. At what temperature would you need 100 ml of water to dissolve 100g NaNO
?
3
10. If 100ml of a saturated solution of NH
Cl at 100C is cooled to 20C, how much precipitate will form?
4
11. If 100 ml of a saturated solution of KNO
at 50C is cooled to 10C, how much precipitate will form?
3
12. 200 ml of a solution contains 10g of KClO
at 15C. The solution is then warmed to 100C. How much
3
additional KClO
may be dissolved?
3
13. How many grams of NaNO
are required to saturate 200ml of water at 10ºC?
3
14. A 100ml solution contains 35g KClO
and 40g of NH
Cl at 80C. As the solution is cooled, which will
3
4
precipitate first?
15. Classify the following solutions as either: saturated, unsaturated or supersaturated (assume that all three
could form supersaturated solutions)
o
a) 40. g of KCl in 100 mL of water at 80
C
o
b) 120. g of KNO
in 100 mL of water at 60
C
3
o
c) 80. g of NaNO
in 100 mL of water at 10
C
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2