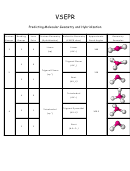
Vsepr Worksheet
ADVERTISEMENT
Gather together the following equipment: 6 small white Styrofoam balls, one large coloured ball, and 6 toothpicks. Complete the chart. Note: when building
molecules use a large coloured Styrofoam ball as the central atom, small white balls as peripheral atoms, and toothpicks to represent bonds. If the molecule
has resonance structures, draw these in the “Lewis structure” box (B), but build only one resonance structure for the “Build molecule” box (C). The bond
angle for a tetrahedral molecule is 109.5°. Other bond angles can be calculated by correctly dividing the 360 degrees of a circle (look at your structure).
B. Lewis structure (use rules
C. Build molecule (see pg 247),
D. Number of
E. Bond angles
F. Name
A. Molecule
for drawing Lewis structures)
sketch, & give bond angles
peripheral atoms
(list all)
(see pg. 247)
H
109.5
1. CH
H
C
H
4
Tetrahedral
109.5°
4
H
2. BeF
2
3. PCl
5
4. CCl
4
–
5. PF
6
6. SO
3
Q1. What does VSEPR stand for? Q2. For molecules that have names associated with their shapes, what is the minimum number of atoms a molecule must have? Explain.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2