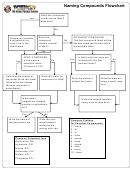
Naming Compounds And Determining Formulas
ADVERTISEMENT
Naming compounds and determining formulas
Binary compounds (two elements)
Name from formula (This is the easy direction.)
The leftmost element of the two (in the periodic table) is first and keeps its name. In ionic compounds this
is the cation or metal. If both elements are in the same family (column), the one with the higher atomic
mass (bottommost in the family) is first and keeps its name.
The other element’s ending is dropped, and the “ide” ending is added. In ionic compounds, this is the
anion.
In ionic compounds, if the cation (metal) is from a group 1A, 2A or 3A (the representative element metal
groups), simply use the metal name and the “ide” ending for the anion.
If the metal is a transition metal (“B” groups in the periodic table), a Roman numeral is used to indicate the
charge on the cation (if there is more than one possible ionic charge for the metal) in this particular
compound. Many of these compounds also have common names. The “ic” ending is used for the metal with
the greater positive charge, “ous” for the smaller charge. See the examples below.
In molecular or nonionic binary compounds, numerical prefixes may be used to denote the number of each
kind of atom.
Examples:
Ionic compounds – representative element metals (groups 1A, 2A and some 3A):
Formula
Name
NaCl
- sodium chloride
MgI
- magnesium iodide
2
Al
O
- aluminum oxide
2
3
Ionic compounds – transition element metals (“B” groups):
Formula
Name (IUPAC)
Common Name
FeCl
- iron (II) chloride
ferrous chloride
2
FeCl
- iron (III) chloride
ferric chloride
3
Cu
O
- copper (I) oxide
cuprous oxide
2
CuO
- copper (II) oxide
cupric oxide
Molecular (nonionic) compounds (usually consisting of nonmetals and/or metalloids):
Formula
Name
BCl
-
boron trichloride
3
H
S
-
hydrogen sulfide or dihydrogen sulfide
2
CO
-
carbon dioxide
2
CO
-
carbon monoxide
P
O
-
diphosphorous pentoxide
2
5
Two caveats:
1.
There are many common names, e.g., water. You just have to memorize them.
2.
Compound and element names are common nouns and are not capitalized except at the beginning of a
sentence.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3