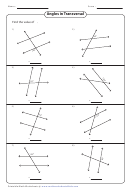
Bonding In Ethene Worksheet
ADVERTISEMENT
C h e m g u i d e – q u e s t i o n s
BONDING IN ETHENE
1. a) Write the electronic structure (using s and p notation) of the carbon atom in its ground
(unexcited) state.
b) When carbon forms its bonds in ethene, an electron on each carbon atom has to be promoted.
Explain what this means, using a diagram if you wish.
c) The electrons in the carbon atoms now hybridise. Describe, using diagrams as necessary, what
happens in this case, naming the type of hybrid orbitals formed.
d) With the help of diagrams, describe the formation of the various bonds in ethene, making clear
the difference between sigma and pi bonds.
2. a) Explain why ethene is a planar molecule with all the bond angles 120°.
b) In ethane, there is free rotation around the bond between the two carbon atoms, but in ethene the
carbon-carbon bond can't rotate unless you raise the temperature quite a lot. Explain why there is a
difference.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1