Extraction Of Metals Iron
ADVERTISEMENT
C h e m g u i d e – q u e s t i o n s
EXTRACTION OF METALS: IRON
It is difficult to ask anything about this which doesn't insult your intelligence! If you are doing a
course for 16 – 18 year old chemistry students, you have almost certainly come across it all in detail
years ago. My apologies!
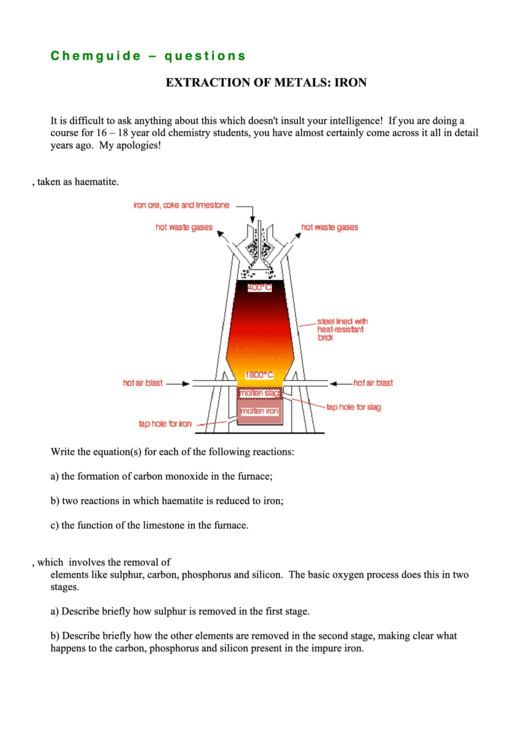
1. The diagram shows the Blast Furnace for the extraction of iron from its ore, taken as haematite.
Write the equation(s) for each of the following reactions:
a) the formation of carbon monoxide in the furnace;
b) two reactions in which haematite is reduced to iron;
c) the function of the limestone in the furnace.
2. Most of the iron emerging from the furnace is converted into steel, which involves the removal of
elements like sulphur, carbon, phosphorus and silicon. The basic oxygen process does this in two
stages.
a) Describe briefly how sulphur is removed in the first stage.
b) Describe briefly how the other elements are removed in the second stage, making clear what
happens to the carbon, phosphorus and silicon present in the impure iron.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2