Alkenes Polymerizations
ADVERTISEMENT
C h e m g u i d e – q u e s t i o n s
ALKENES: POLYMERISATION
I am not asking for the conditions for the various polymerisation processes mentioned on the
Chemguide page because these don't involve any understanding – just last minute learning of
whatever is likely to come up in your exam.
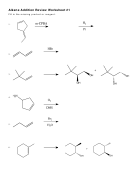
1. a) Draw the structure of the monomer you would use to make this polymer:
-CH
CHCH
CHCH
CHCH
CHCH
CH-
2
2
2
2
2
CN
CN
CN
CN
CN
b) Draw the structure of the polymer you would get by polymerising the monomer C
H
CH=CH
,
6
5
2
showing at least three repeating units.
2. Poly(ethene) comes in two types – low density poly(ethene) (LDPE) and high density poly(ethene)
(HDPE). The difference lies in the amount of branching in the polymer chains.
When LDPE is made, lots of branches form along the chains, whereas HDPE has very little
branching. Explain how the amount of branching affects
a) the melting points of the two types;
b) the strength of the two types, and hence their uses;
c) the density of the two types.
3. a) Poly(propene) comes in a number of different forms, but the commonest one is isotactic
poly(propene). It has the structure (taken from the Chemguide page):
Explain what this diagram shows about the arrangement of the CH
groups along the chain.
3
b) Another form is atactic poly(propene) in which the arrangement of the CH
groups is random.
3
For example:
Explain why isotactic poly(propene) is a more useful polymer than atactic poly(propene).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2