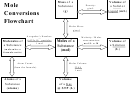
Mole Concept Qualifier
ADVERTISEMENT
Name_______________________________
Chemistry I-Honors
Mole Concept Qualifier
Complete the following questions. Show all work or no credit will be given.
Explain the reasoning behind why you wrote what formula for which name-box this so I can see it.
1._______________________
33.4 liters of carbon monoxide gas at STP contains how many
molecules of this gas?
2.________________________
How many cations are in 11.0 milligrams of copper(II) arsenate?
3.________________ What is the number of moles of oxygen atoms that can be released from 49.0 grams of
carbonic acid?
4a._________________ If there are 2.445 grams of chlorine present in iron (II) perchlorate tetrahydrate, how
many water molecules are also present in this same sample?
4b._________________ Using the same information in the previous question, how many oxygen atoms are
present in the sample?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4