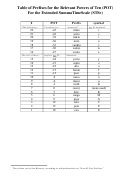
Table Of Standard Reduction Potentials
ADVERTISEMENT
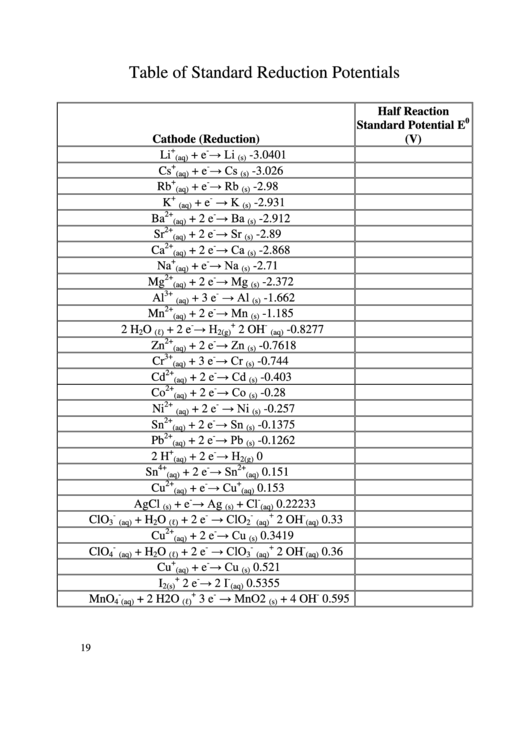
Table of Standard Reduction Potentials
Half Reaction
0
Standard Potential E
(V)
Cathode (Reduction)
+
-
Li
+ e
→ Li
-3.0401
(aq)
(s)
+
-
Cs
+ e
→ Cs
-3.026
(aq)
(s)
+
-
→ Rb
Rb
+ e
-2.98
(aq)
(s)
+
-
→ K
K
+ e
-2.931
(aq)
(s)
2+
-
Ba
+ 2 e
→ Ba
-2.912
(aq)
(s)
2+
-
Sr
+ 2 e
→ Sr
-2.89
(aq)
(s)
2+
-
Ca
+ 2 e
→ Ca
-2.868
(aq)
(s)
+
-
Na
+ e
→ Na
-2.71
(aq)
(s)
2+
-
→ Mg
Mg
+ 2 e
-2.372
(aq)
(s)
3+
-
Al
+ 3 e
→ Al
-1.662
(aq)
(s)
2+
-
Mn
+ 2 e
→ Mn
-1.185
(aq)
(s)
-
+
-
2 H
O
+ 2 e
→ H
2 OH
-0.8277
2
(ℓ)
2 (g)
(aq)
2+
-
Zn
+ 2 e
→ Zn
-0.7618
(aq)
(s)
3+
-
Cr
+ 3 e
→ Cr
-0.744
(aq)
(s)
2+
-
→ Cd
Cd
+ 2 e
-0.403
(aq)
(s)
2+
-
Co
+ 2 e
→ Co
-0.28
(aq)
(s)
2+
-
Ni
+ 2 e
→ Ni
-0.257
(aq)
(s)
2+
-
Sn
+ 2 e
→ Sn
-0.1375
(aq)
(s)
2+
-
Pb
+ 2 e
→ Pb
-0.1262
(aq)
(s)
+
-
2 H
+ 2 e
→ H
0
(aq)
2 (g)
4+
-
2+
Sn
+ 2 e
→ Sn
0.151
(aq)
(aq)
2+
-
+
Cu
+ e
→ Cu
0.153
(aq)
(aq)
-
-
→ Ag
AgCl
+ e
+ Cl
0.22233
(s)
(s)
(aq)
-
-
-
+
-
ClO
+ H
O
+ 2 e
→ ClO
2 OH
0.33
3
(aq)
2
(ℓ)
2
(aq)
(aq)
2+
-
Cu
+ 2 e
→ Cu
0.3419
(aq)
(s)
-
-
-
+
-
ClO
+ H
O
+ 2 e
→ ClO
2 OH
0.36
4
(aq)
2
(ℓ)
3
(aq)
(aq)
+
-
Cu
+ e
→ Cu
0.521
(aq)
(s)
+
-
-
→ 2 I
I
2 e
0.5355
2 (s)
(aq)
-
+
-
-
→ MnO2
MnO
+ 2 H2O
3 e
+ 4 OH
0.595
4
(aq)
(ℓ)
(s)
19
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2